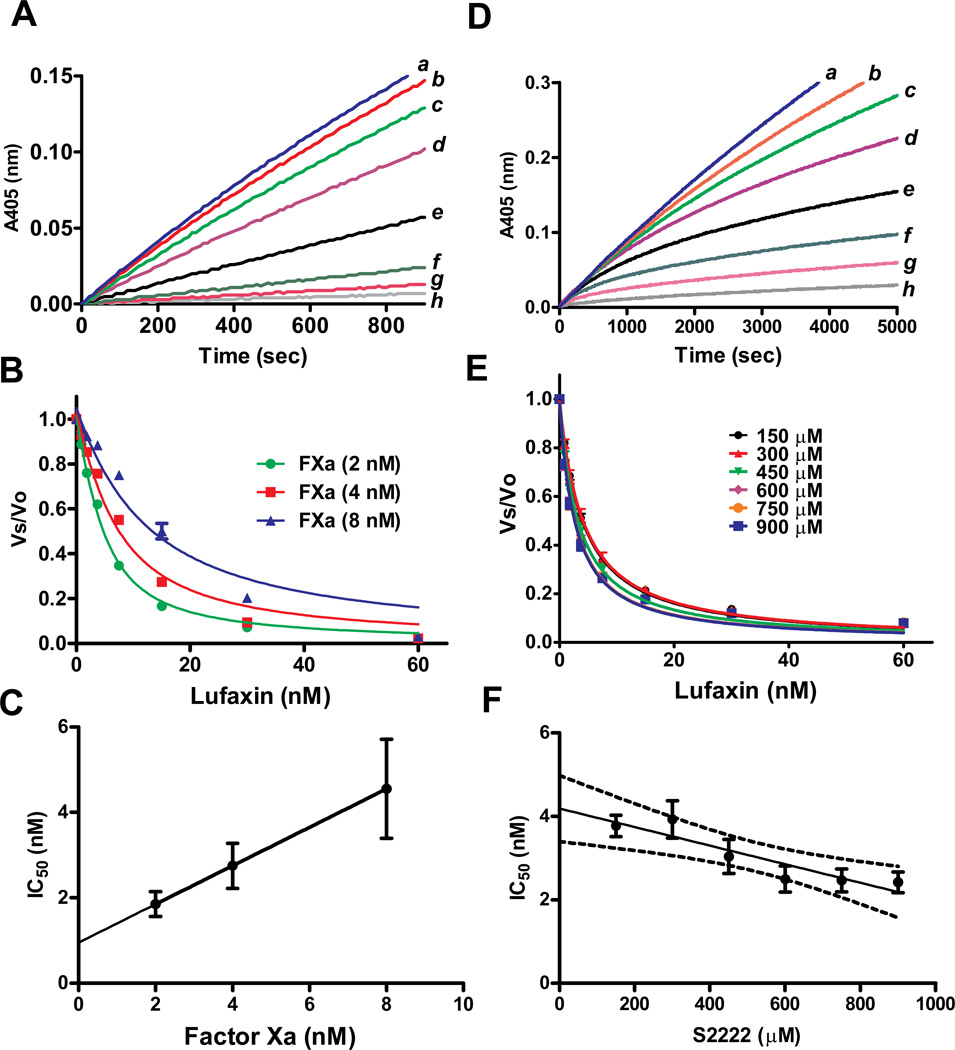
Figure 3.
Lufaxin is a tight and slow inhibitor of FXa. A, Typical progress curves for FXa-mediated S2222 hydrolysis in the absence (curve a) and presence of Lufaxin (curves b, 0.93 nM; c, 1.8 nM; d, 3.75 nM; e, 7.5 nM; f, 15 nM; g, 30 nM, and h, 60 nM). Reactions started with addition of S2222 (250 µM) to a mixture containing Lufaxin incubated for 1 hour with FXa (2 nM). Substrate hydrolysis was followed for 2 hours at 37°C, at 405 nm. B, The experiment was performed as in A but FXa concentrations were 2, 4, and 8 nM. The ratio of Vs/Vo was plotted against Lufaxin concentration and data fitted with the Morrison equation to calculate the IC50 at each enzyme concentration. C, Plot of IC50 and FXa produces a straight line typical of tight inhibitors. D, Slow-type inhibition of FXa by Lufaxin. Typical progress curves for FXa-mediated S2222 (1 mM) hydrolysis in the absence (curve a) and presence of Lufaxin (curves b, 0.93 nM; c, 1.8 nM; d, 3.75 nM; e, 7.5 nM; f, 15 nM; g, 30 nM and h, 60 nM). Reactions started with addition of FXa (0.5 nM) to a mixture containing Lufaxin and S2222 (1 mM). Substrate hydrolysis was followed for 2 hours at 37°C and 405 nm. E, Experiments were performed as in D but S2222 concentrations were 150, 300, 450, 600, 750, 900 µM. The ratio Vs/Vo obtained between 45 – 60 min was plotted against S2222 concentration and data fitted with the Morrison equation to calculate the IC50 at each substrate concentration. F, Plot IC50 and S2222 concentrations. Value were fitted by linear regression. The pattern of curves is typical for non competitive inhibitors. Six experiments were performed, and each data point is the average of duplicate determinations.