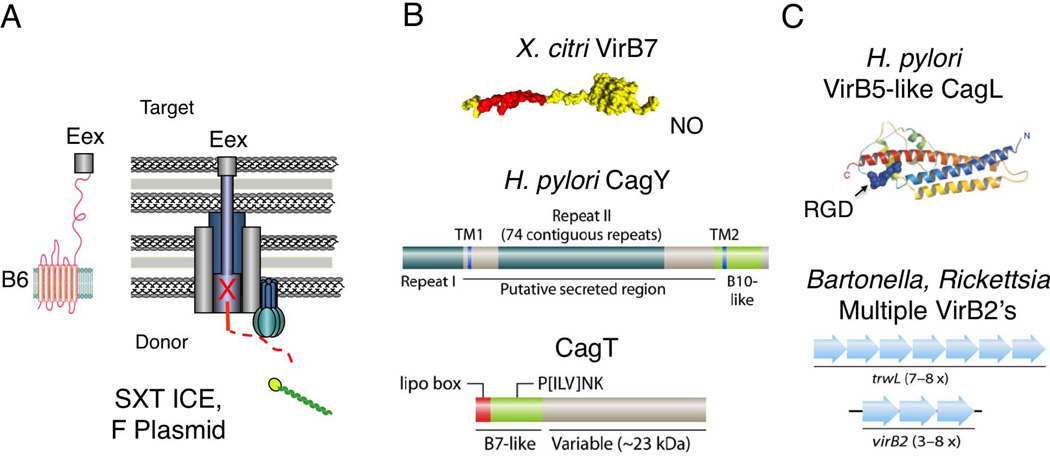
Fig. 11.
Examples of T4SS subunit acquisitions of structural folds for modulation of target cell interactions. (A) Some VirB6-like subunits possess C-terminal extensions implicated in surface exposure, e.g. Rickettsia spp. VirB6 (not shown), or extension through the T4SS channel and the recipient cell to the inner membrane where contact is established with the entry exclusion protein Eex, e.g. V. cholera SXT TraG and F plasmid TraG, which prevents redundant transfer to donor cells. (B) Several T4SSs possess variant forms of the VirB7/VirB9/VirB10 core complex subunits. X. citri VirB7XAC2622, a VirB7-like lipoprotein with an N-terminal (red) domain, implicated in binding VirB9XAC2620, and a C-terminal N0 domain (globular domain in yellow), which could mediate binding to target cells or substrate translocation through the T4SS. H. pylori VirB7-like CagT and VirB10-like CagY possess repeat domains implicated in forming a surface-variable sheath structure. (C) H. pylori VirB5-like CagL has an RGD motif implicated in binding of the β-integrin receptor on the host cell. Bartonella and Rickettsia spp. code for multiple copies of VirB2-like pilins, TrwL and VirB2, respectively, implicated in forming surface-variable pilins for immunomodulation. The X. citri VirB7 X-ray structure is reprinted with permission from (Souza et al., 2011). The modeled structure of VirB5-like CagL is reprinted with permission from (Backert et al., 2008). The schematics in Figs. B and C are reprinted with permission from (Alvarez-Martinez & Christie, 2009).