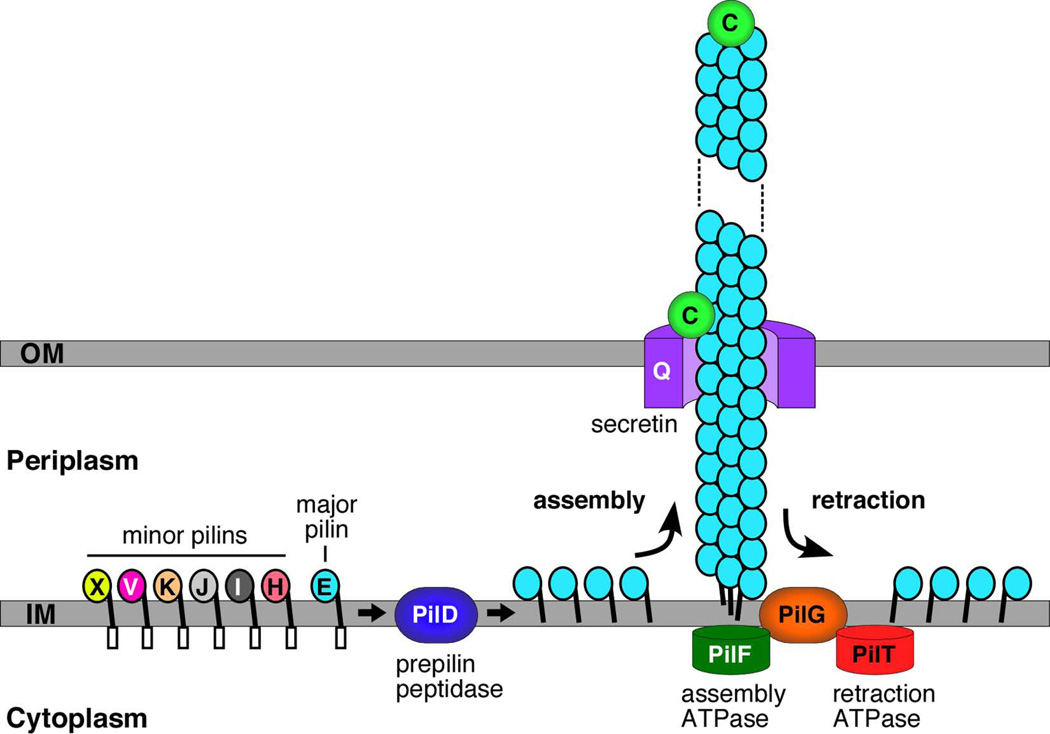
Fig. 5.
Model for biogenesis of T4P by N. meningitidis. The major (PilE) and minor (PilH, I, J, K, V and X) pilins are anchored in the inner membrane by their hydrophobic N-termini, with the conserved prepilin leader sequence exposed to the cytoplasm and globular head domain exposed to the periplasm. The leader sequence is cleaved and the mature pilins are N-methylated by the PilD prepilin peptidase. Processed pilin subunits are assembled into the pilus fiber from the periplasmic face of the inner membrane. PilF is the cytoplasmic ATPase that powers pilus assembly. The PilQ secretin provides the channel for secretion of the fiber across the outer membrane to the cell surface. Retraction of the pilus fiber is powered by the PilT cytoplasmic ATPase. During retraction, pilins are disassembled from the base of the pilus fiber and enter back into the inner membrane. The PilC protein functions as a tip-located adhesin, and also localizes to the outer membrane where it regulates pilus retraction.