Figure 1.
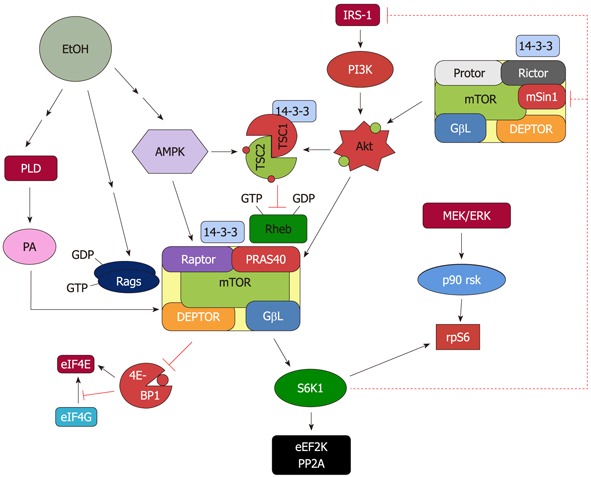
Proposed model for the regulation of mammalian target of rapamycin complex 1 and mammalian target of rapamycin complex 2 in response to EtOH. EtOH decreases the activity of mammalian target of rapamycin complex (mTORC)1 toward its substrates 4E binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1). This process is mediated via multiple signaling routes including AMP-activated protein kinase (AMPK), phosphoinositol-3 kinase (PI3K)/Akt, phospholipase D (PLD) and Rag GTPases. The role each of these upstream regulators plays in affecting mTORC1 function is discussed in the text. The decrease in S6K1 phosphorylation with EtOH signals to mTORC2 as part of a feedback loop. As such, EtOH increases mTORC2 activity toward Akt, which then enhances phosphorylation of its substrate proline rich Akt substrate of 40 kDa (PRAS40).
