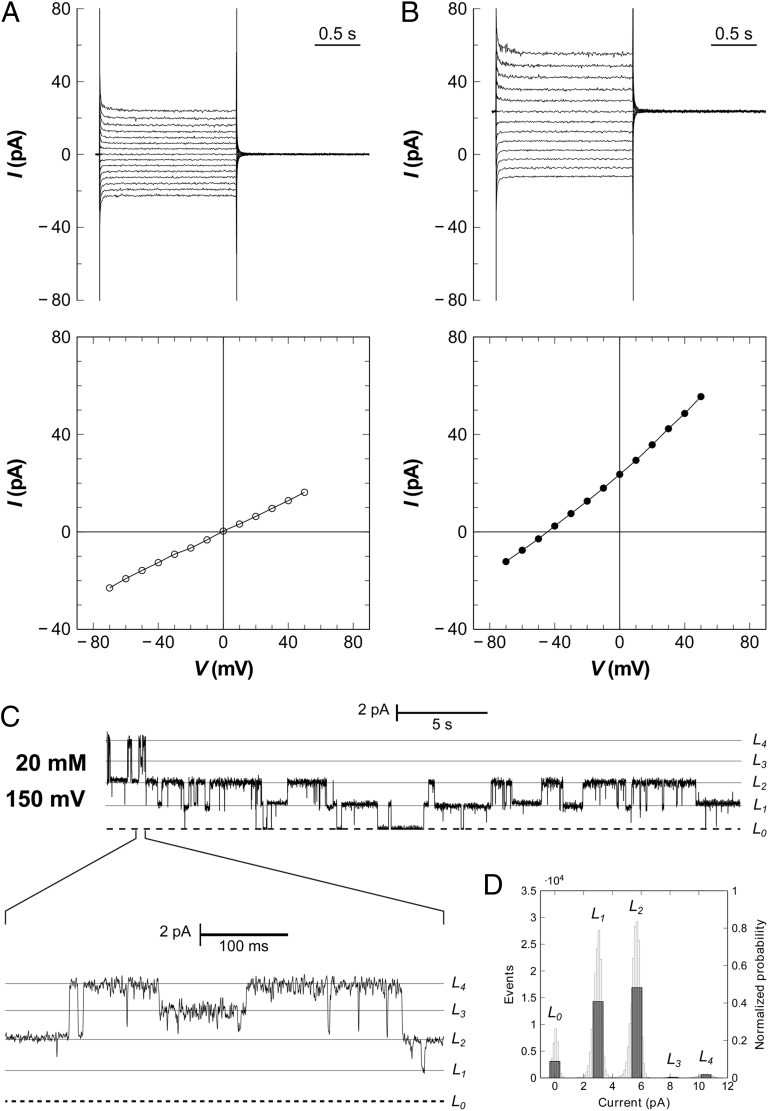
Fig. 2.
Electrophysiology of FocA reconstituted in planar lipid bilayers. (A) In the presence of symmetric formate concentrations (20/20 mM), the reversal potential derived from a current-voltage plot is zero. Macroscopic currents are shown above and the respective current–voltage diagram below. (B) Macroscopic currents obtained with a formate gradient of 100 mM (cis side) to 19.6 mM (trans side) yield a reversal potential of –42 mV at 298 K, matching precisely the expected Nernst potential. The membrane for the experiment in A and B contained at least 22 channels, or five pentamers, consistent with the observed single-channel currents. (C) At lower protein concentrations, single-channel opening and closing events are observed, with a current of 2.6 pA per protomer at a membrane potential of +150 mV and symmetric formate concentrations of 20 mM. (D) All-point histogram for the current trace in C. The L1 and L2 states are far higher populated than the others, indicating subunit cooperativity for FocA.