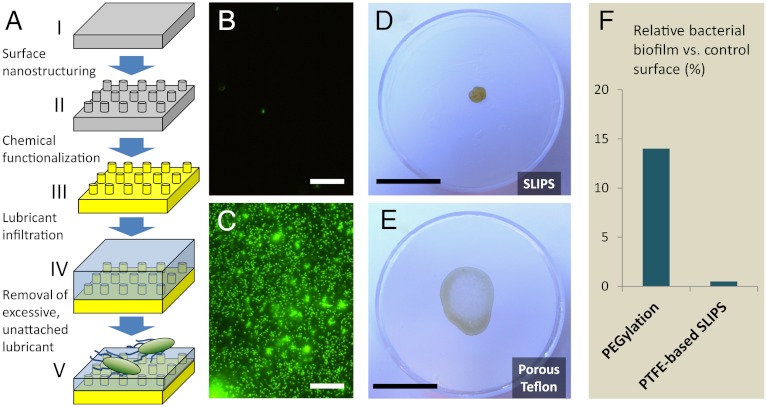
Fig. 1.
SLIPS preparation and biofilm attachment to the surfaces investigated in this study. (A) Schematic of slippery liquid-infused porous surface (SLIPS) material concept. A flat substrate (i) is nano-patterned or roughened (ii), chemically functionalized (iii), and infused with a compatible lubricating liquid (iv), of which the excess is removed (v). Porous Teflon substrates used in this study are stage III-ready for the infiltration with perfluorinated lubricants. The two-part system presents a “slippery” surface of highly immiscible immobilized liquid to bacteria. (B–C) Fluorescence micrographs of attached bacteria following 48 h incubation of P. aeruginosa biofilm on SLIPS (B) and superhydrophobic PTFE (C). Scale bar = 30 μm. (D–E) Remains of an evaporated drop of P. aeruginosa biofilm-forming culture on SLIPS (D) and superhydrophobic PTFE (E). The poorly attached biofilm on the SLIPS substrate cleanly retracts from the surface as it evaporates (see Movie S3), leaving behind a small, easily removable pellet (see Movie S4). In contrast, biofilm grown on the PTFE and microstructured superhydrophobic silicon (Movie S1) shows complete wetting of the surface and leaves behind a slimy, firmly attached coffee ring. Scale bar = 2 cm. (F) Comparison of biofilm attachment to our SLIPS substrate after 7 d and to a PEGylated substrate after 5 h, as reported in (44). Even assuming a best-case scenario in which its 5 h PEG performance can be maintained at 7 d without desorption or masking of surface chemistry, the 0.4% relative attachment to SLIPS represents a > 30 times improvement.