Abstract
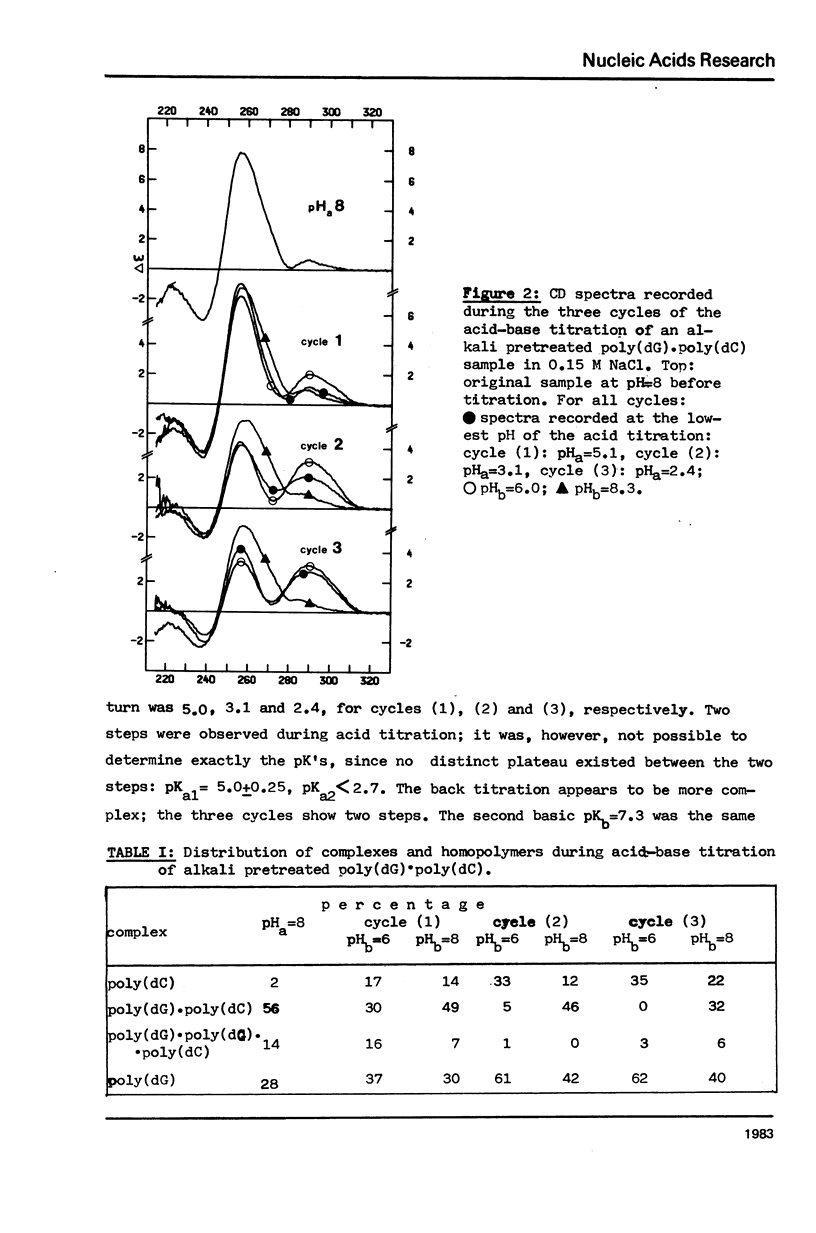
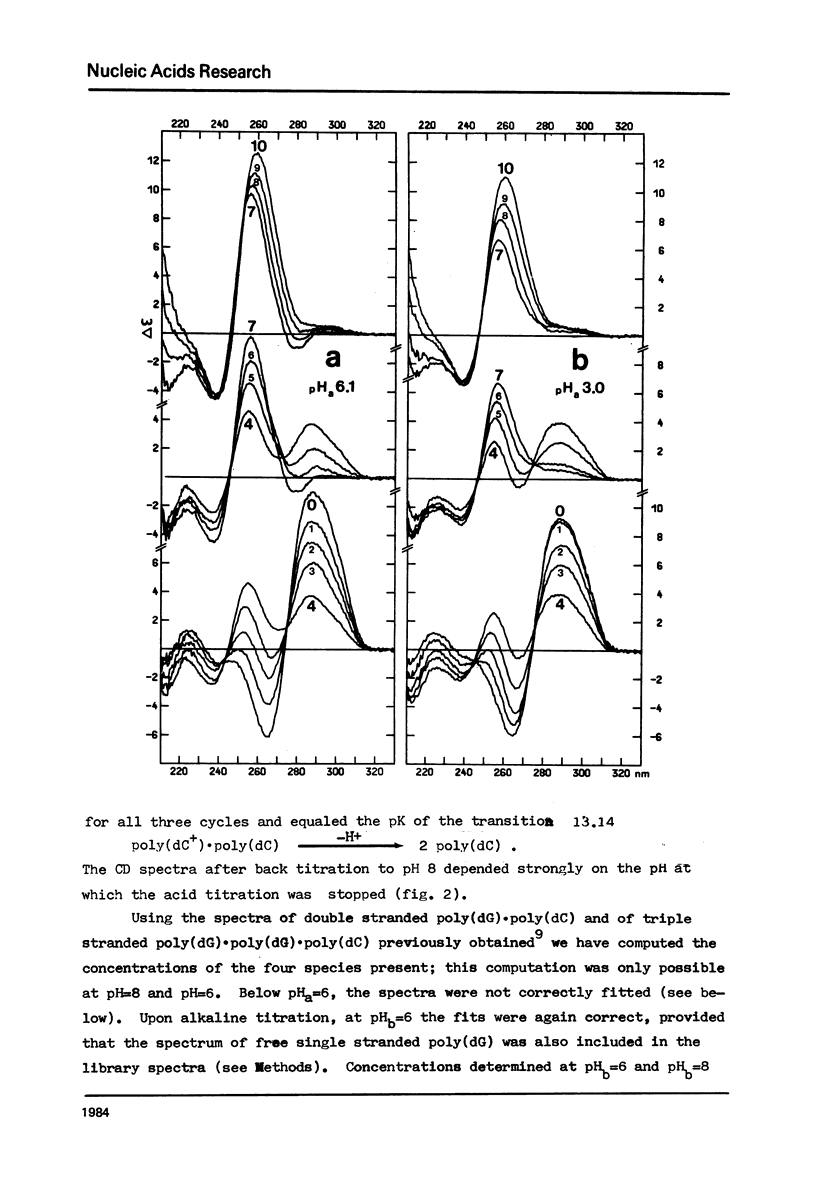
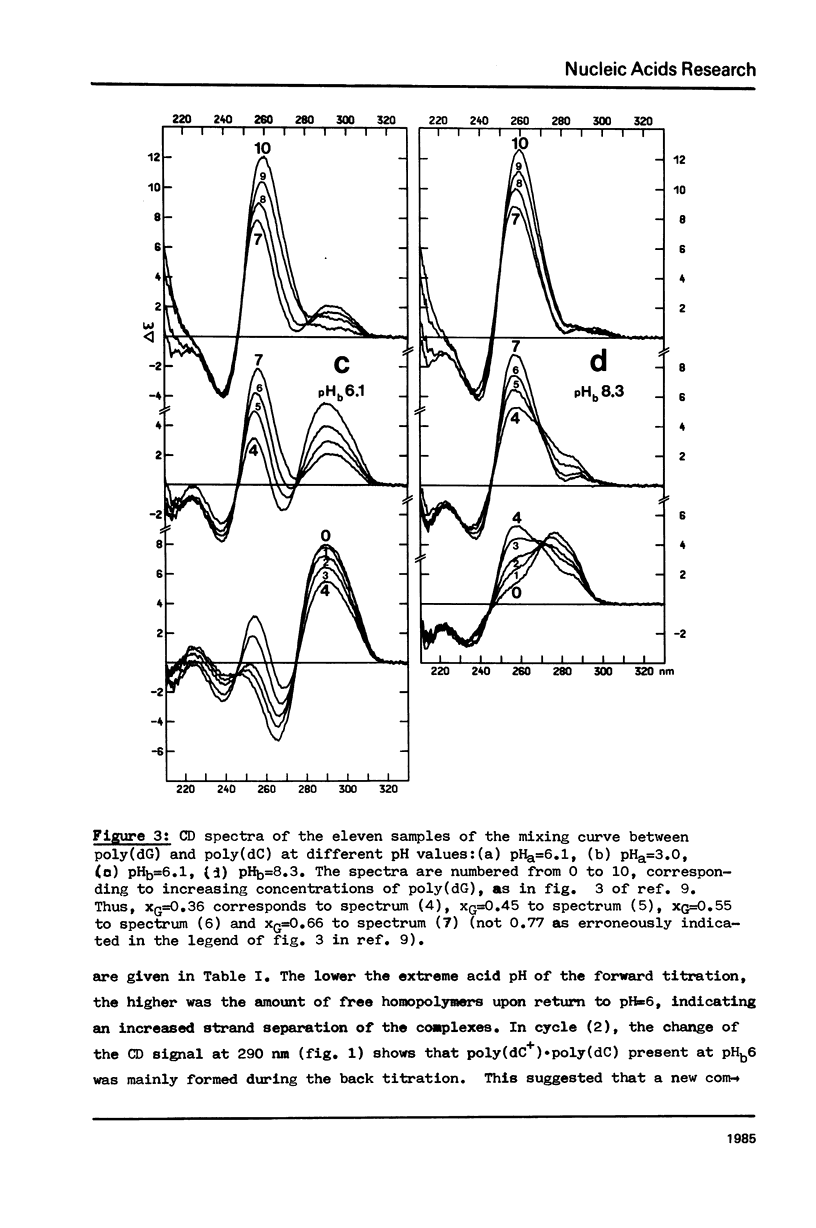
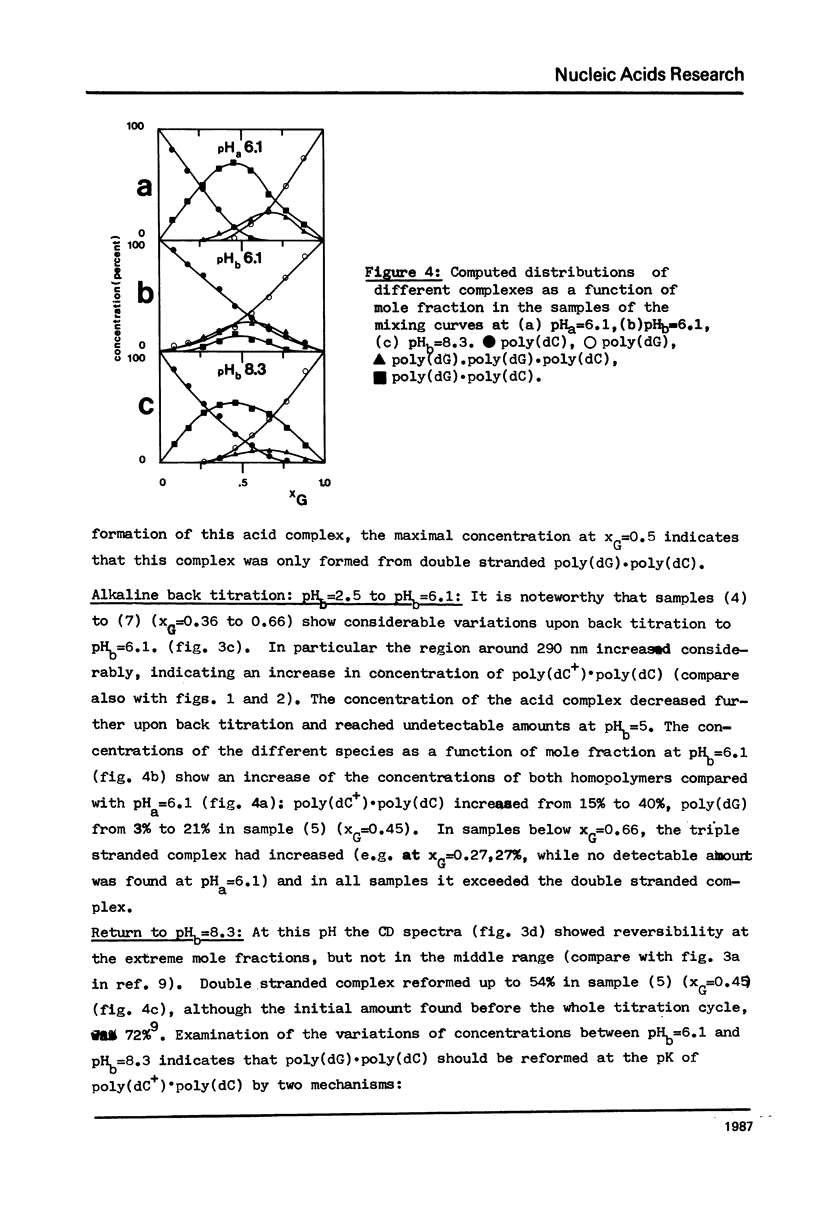
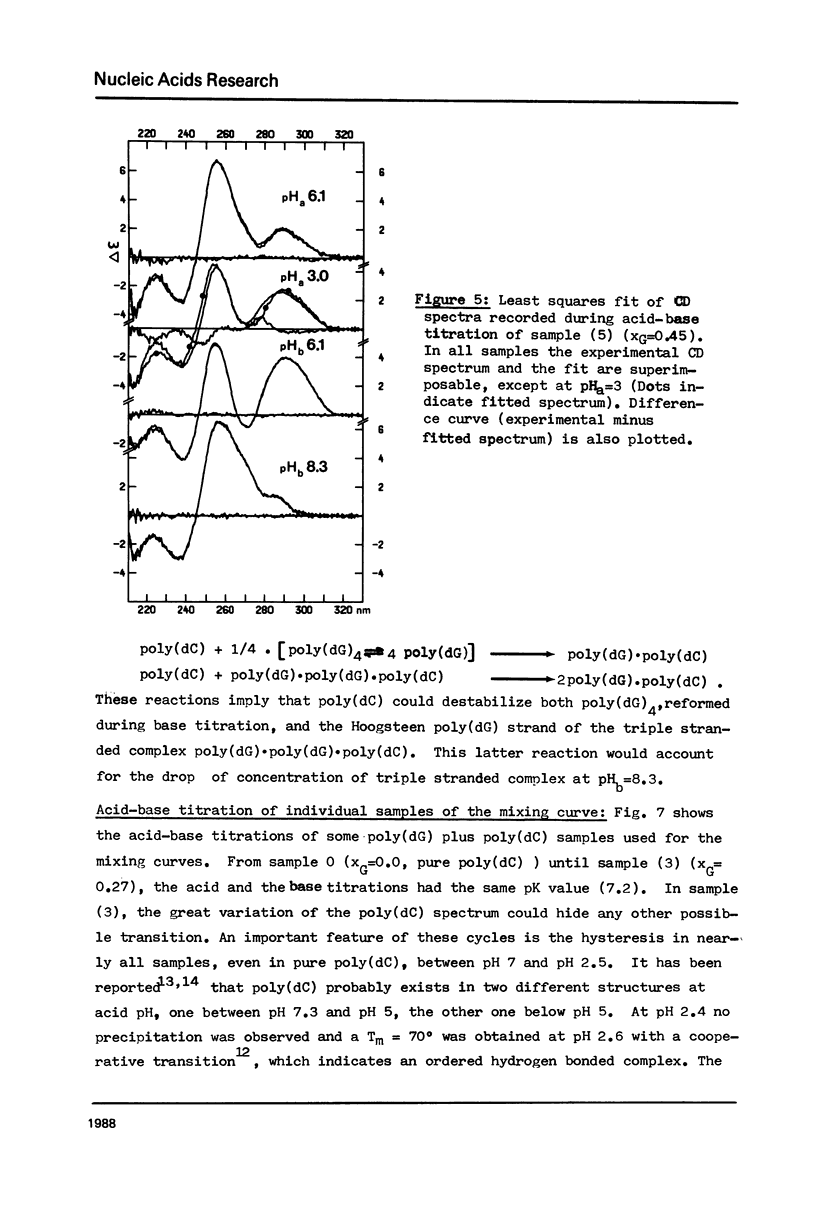
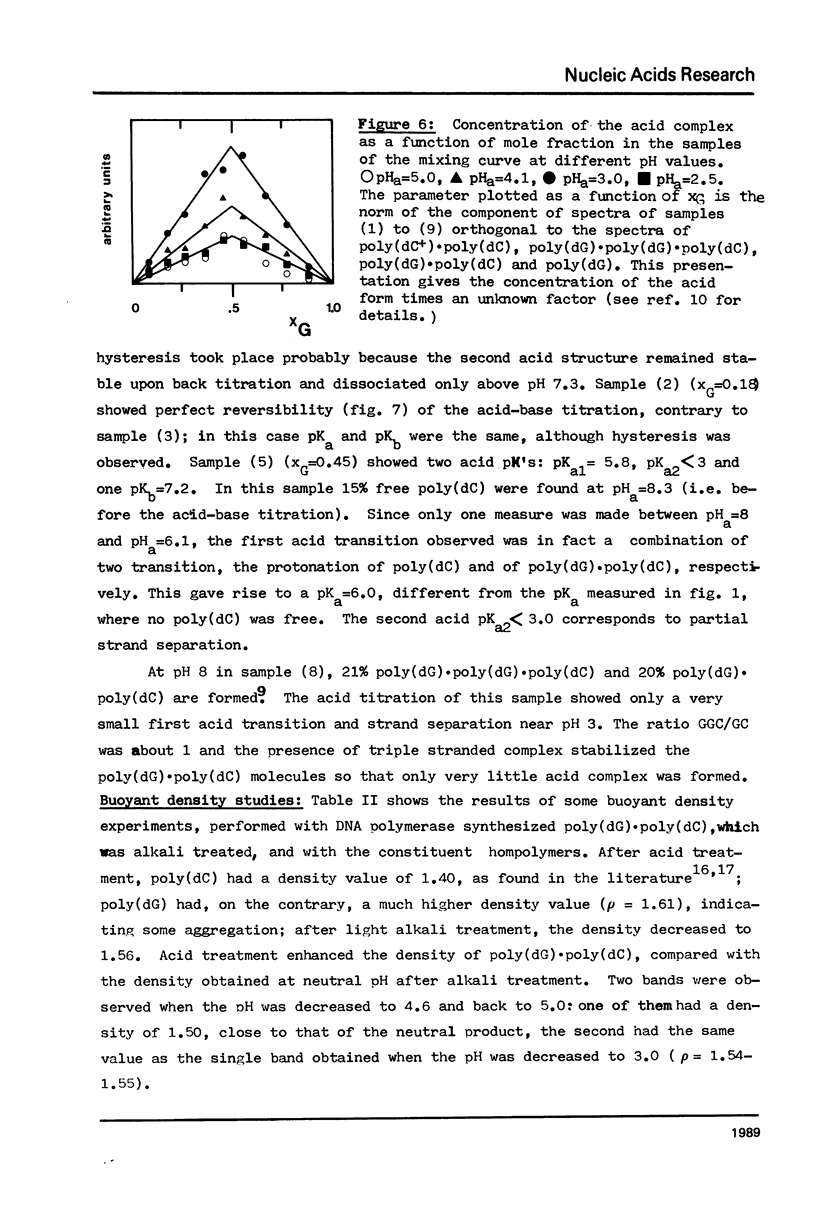
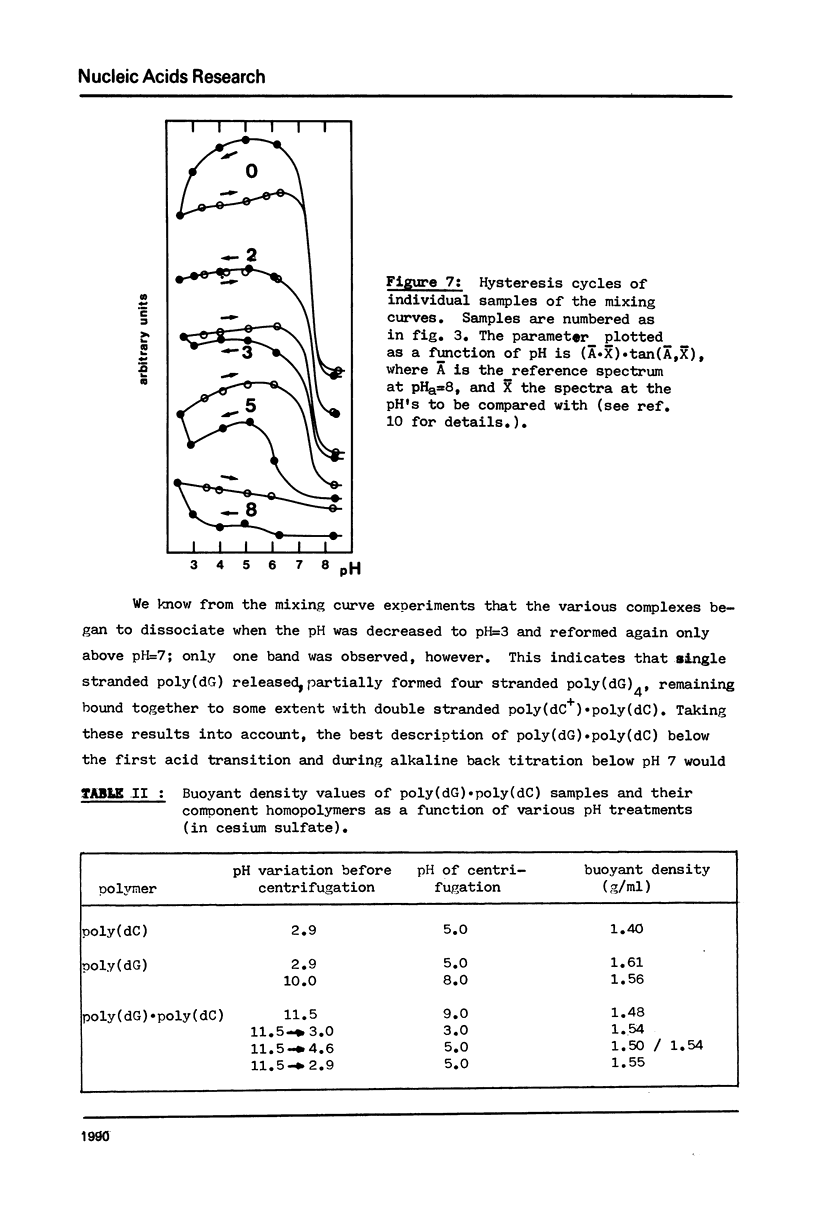
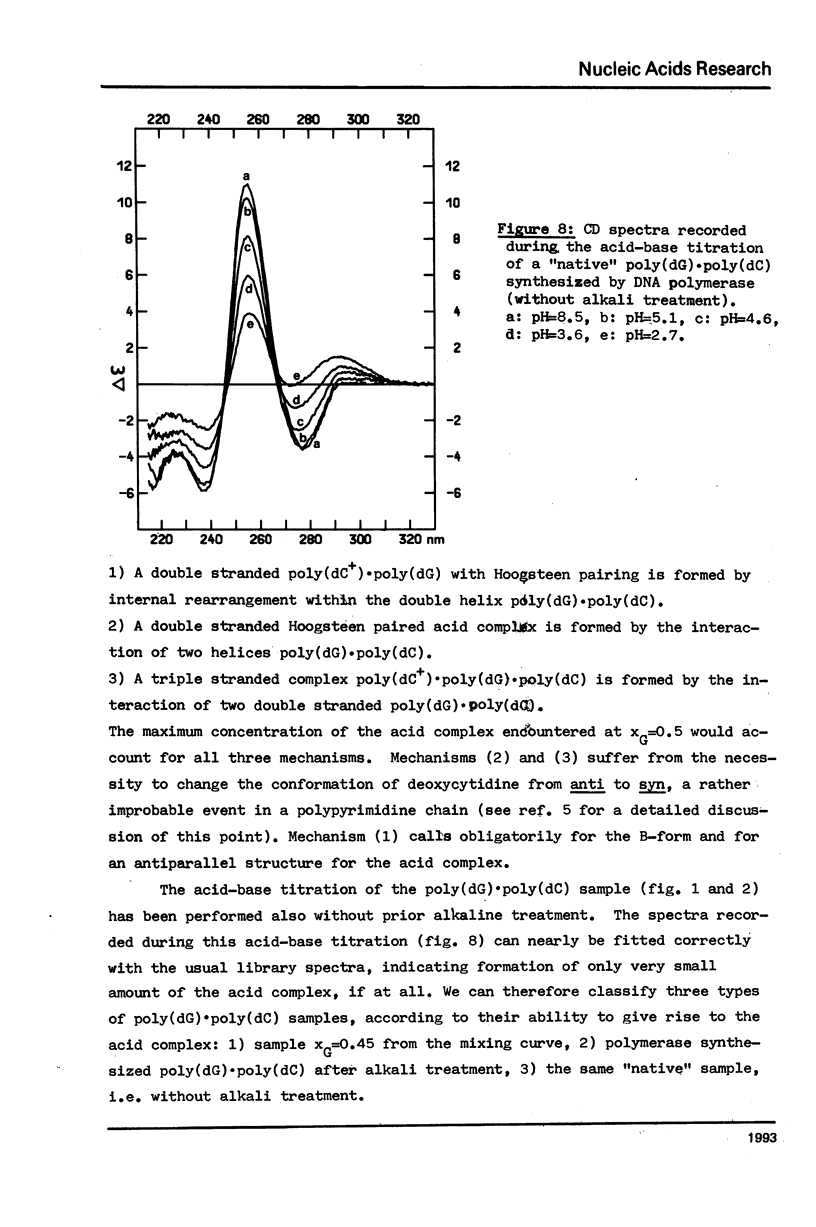
The acid-base titration (pH 8 → pH 2.5 → pH 8) of eleven mixing curve samples of the poly(dG) plus poly(dC) system has been performed in 0.15 M NaCl. Upon protonation, poly(dG)·poly(dC) gives rise to an acid complex, in various amounts according to the origin of the sample. We have established that the hysteresis of the acid-base titration is due to the non-reversible formation of an acid complex, and the liberation of the homopolymers at the end of the acid titration and during the base titration: the homopolymer mixtures remain stable up to pH 7. A 1G:1C stoichiometry appears to be the most probable for the acid complex, a 1G:2C stoichiometry, as found in poly(C+)·poly(I)·poly(C) or poly(C+)·poly(G)·poly(C), cannot be rejected. In the course of this study, evidence has been found that the structural consequences of protonation could be similar for both double stranded poly(dG)·poly(dC) and G-C rich DNA's: 1) protonation starts near pH 6, dissociation of the acid complex of poly(dG)·poly(dC) and of protonated DNA take place at pH 3; 2) the CD spectrum computed for the acid polymer complex displays a positive peak at 255 nm as found in the acid spectra of DNA's; 3) double stranded poly(dG)·poly(dC) embedded in triple-stranded poly(dG)·poly(dG)·poly(dC) should be in the A-form and appears to be prevented from the proton induced conformational change. The neutral triple stranded poly(dG)·poly(dG)·poly(dC) appears therefore responsible, although indirectly, for the complexity and variability of the acid titration of poly(dG)·poly(dC) samples.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B. A., Grossman L., Fasman G. D. Single-stranded oligomers and polymers of cytidylic and 2'-deoxycytidylic acids: comparative optical rotatory studies. Proc Natl Acad Sci U S A. 1967 Feb;57(2):423–430. doi: 10.1073/pnas.57.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott S., Bond P. J., Selsing E., Smith P. J. Models of triple-stranded polynucleotides with optimised stereochemistry. Nucleic Acids Res. 1976 Oct;3(10):2459–2470. doi: 10.1093/nar/3.10.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAMBERLIN M. J., PATTERSON D. L. PHYSICAL AND CHEMICAL CHARACTERIZATION OF THE ORDERED COMPLEXES FORMED BETWEEN POLYINOSINIC ACID, POLYCYTIDYLIC ACID AND THEIR DEOXYRIBO-ANALOGUES. J Mol Biol. 1965 Jun;12:410–428. doi: 10.1016/s0022-2836(65)80264-9. [DOI] [PubMed] [Google Scholar]
- Courtois Y., Fromageot P., Guschlbauer W. Protonated polynucleotide structures. 3. An optical rotatory dispersion study of the protonation of DNA. Eur J Biochem. 1968 Dec 5;6(4):493–501. doi: 10.1111/j.1432-1033.1968.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Gray D. M. A circular dichroism study of poly dG, poly dC, and poly dG:dC. Biopolymers. 1974;13(10):2087–2102. doi: 10.1002/bip.1974.360131011. [DOI] [PubMed] [Google Scholar]
- Guschlbauer W. Protonated polynucleotide structures. 16. Thermodynamics of the melting of the acid form of polycytidylic acid. Nucleic Acids Res. 1975 Mar;2(3):353–360. doi: 10.1093/nar/2.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INMAN R. B., BALDWIN R. L. HELIX--RANDOM COIL TRANSITIONS IN DNA HOMOPOLYMER PAIRS. J Mol Biol. 1964 Apr;8:452–469. doi: 10.1016/s0022-2836(64)80003-6. [DOI] [PubMed] [Google Scholar]
- Marck C., Thiele D. Poly(dG).poly(dC) at neutral and alkaline pH: the formation of triple stranded poly(dG).poly(dG).poly(dC). Nucleic Acids Res. 1978 Mar;5(3):1017–1028. doi: 10.1093/nar/5.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikke R., Zmudzka B. Oligo(dG)12-18 aggregates result in non-homogeneity of oligo(dG)12-18.poly(C) type primer-template. Nucleic Acids Res. 1977 Apr;4(4):1111–1122. doi: 10.1093/nar/4.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan A. R., Wells R. D. Specificity of the three-stranded complex formation between double-stranded DNA and single-stranded RNA containing repeating nucleotide sequences. J Mol Biol. 1968 Oct 14;37(1):63–80. doi: 10.1016/0022-2836(68)90073-9. [DOI] [PubMed] [Google Scholar]
- Sarocchi-Landousy M. T., Haas B. L., Guschlbauer W. Interaction of oligoribocytidylates with T7 DNA in neutral and acid media. Biochemistry. 1977 Dec 13;16(25):5414–5420. doi: 10.1021/bi00644a002. [DOI] [PubMed] [Google Scholar]
- Sarocchi M. T., Darlix J. L. A spectroscopic approach to DNA transcription and protein binding. Eur J Biochem. 1974 Aug 1;46(3):481–489. doi: 10.1111/j.1432-1033.1974.tb03641.x. [DOI] [PubMed] [Google Scholar]
- TS'O P. O., HELMKAMP G. K., SANDER C. Secondary structures of nucleic acids in organic solvents. II. Optical properties of nucleotides and nucleic acids. Biochim Biophys Acta. 1962 May 14;55:584–600. doi: 10.1016/0006-3002(62)90837-5. [DOI] [PubMed] [Google Scholar]
- Thiele D., Guschlbauer W. Polynucléotides protonés. VII. Transitions thermiques entre differents complexes de l'acide polyinosinique et de l'acide polycytidylique en milieu acide. Biopolymers. 1969;8(3):361–378. doi: 10.1002/bip.1969.360080307. [DOI] [PubMed] [Google Scholar]
- Thiele D., Guschlbauer W. Protonated polynucleotide structures. IX. Disproportionation of poly (G)-poly (C) in acid medium. Biopolymers. 1971;10(1):143–157. doi: 10.1002/bip.360100111. [DOI] [PubMed] [Google Scholar]
- Thiele D., Marck C., Schneider C., Guschlbauer W. Protonated polynucleotides structures - 23. The acid-base hysteresis of poly(dG).poly(dC). Nucleic Acids Res. 1978 Jun;5(6):1997–2012. doi: 10.1093/nar/5.6.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells R. D., Larson J. E., Grant R. C., Shortle B. E., Cantor C. R. Physicochemical studies on polydeoxyribonucleotides containing defined repeating nucleotide sequences. J Mol Biol. 1970 Dec 28;54(3):465–497. doi: 10.1016/0022-2836(70)90121-x. [DOI] [PubMed] [Google Scholar]
- Zimmer C., Luck G., Venner H., Fric J. Studies on the conformation of protonated DNA. Biopolymers. 1968 Apr;6(4):563–574. doi: 10.1002/bip.1968.360060410. [DOI] [PubMed] [Google Scholar]
- Zmudzka B., Bollum F. J., Shugar D. Poly-5-methyldeoxycyctidylic acid and some alkylamino analogs. Biochemistry. 1969 Jul;8(7):3049–3059. doi: 10.1021/bi00835a054. [DOI] [PubMed] [Google Scholar]


