Abstract
The recent emergence of carbapenemase-producing Enterobacteriaceae strains represents a major threat for hospitalized patients. We document the dissemination and control of carbapenemase-producing Klebsiella pneumoniae clones in a Greek hospital. During a 3-year study period (January 2009 to December 2011), carbapenemase-producing K. pneumoniae strains were isolated from clinical samples from 73 individual patients. Phenotyping and molecular testing confirmed that 52 patients were infected with K. pneumoniae carbapenemase 2 (KPC-2) producers, 12 were infected with VIM-1 producers, and the remaining 9 were infected with isolates producing both KPC-2 and VIM-1 enzymes. Twenty-eight of these clinical cases were characterized as imported health care associated, and 23 of these were attributed to KPC producers and 5 were attributed to KPC and VIM producers. The remaining 45 cases were deemed hospital acquired. In the second year of the study, intensified infection control intervention was implemented, followed by active surveillance and carrier isolation in the third year. The incidence of carbapenemase-producing K. pneumoniae patient cases decreased from 0.52/1,000 patient days in 2009 to 0.32/1,000 patient days in 2010 (P = 0.075). Following these additional infection control measures, the incidence fell to 0.21/1,000 patient days in 2011 and differed significantly from that in 2009 (P = 0.0028). Despite the fact that the imported cases of carbapenemase-producing K. pneumoniae were equally distributed over this 3-year period, the incidence of hospital-acquired cases decreased from 0.36/1,000 patient days in 2009 to 0.19/1,000 patient days in 2010 (P = 0.058) and to 0.1/1,000 patient days in 2011 (P = 0.0012). Our findings suggest that rigorous infection control measures and active surveillance can effectively reduce the incidence of secondary transmission due to KPC-producing pathogens.
INTRODUCTION
Carbapenems are considered a last-resort antibiotic regimen for the treatment of infections caused by multidrug-resistant Enterobacteriaceae. However, over the last decade, carbapenem resistance, attributed to the production of carbapenem-hydrolyzing β-lactamases, has steadily increased among Enterobacteriaceae isolates (12, 19). Ambler class B metallo-β-lactamases (MBLs) have emerged among Enterobacteriaceae in the Far East and southern Europe, while Ambler class A Klebsiella pneumoniae carbapenemase (KPC) types initially spread in the northeastern United States and thereafter in several other large geographic regions (19). One particular concern is the association of the latter enzymes with internationally successful K. pneumoniae clones, thus creating an endemicity situation in regions of the United States, Israel, Italy, and Greece, as well as in countries of South America and the Far East (1, 14, 18, 26). KPC-producing members of the family Enterobacteriaceae have also been associated with high mortality rates, particularly among critically ill patients with a history of prolonged hospitalization (16, 20, 25, 27, 33). These facts strongly suggest a need for the implementation of adequate preventive measures in order to effectively contain the spread of such pathogens.
Several outbreaks involving KPC-producing K. pneumoniae strains have been recently reported in Greek hospitals (11, 23, 27). Furthermore, the emergence of strains which coproduce the KPC- and VIM-type MBL genes has also been documented (11, 24). In January 2008, a KPC-producing K. pneumoniae isolate was detected for the first time in our hospital (29). Approximately a year later, in January 2009, the increase noted in the admissions of patients with community onset infections due to KPC-producing K. pneumoniae strains initiated a 3-year study. We describe here the emergence of infections due to carbapenemase-producing K. pneumoniae strains and the effectiveness of the infection control intervention measures which were taken to restrain the dissemination of imported KPC clones in our hospital facilities.
MATERIALS AND METHODS
Bacterial isolates and patients.
During the study period (January 2009 to December 2011), all carbapenem-resistant (imipenem and/or meropenem MIC, >2 μg/ml) K. pneumoniae isolates recovered from clinical samples from patients hospitalized in the Serres General Hospital, Serres, Greece, were prospectively collected. The Serres General Hospital is a 410-bed community-based hospital serving a population of more than 200,000 inhabitants. It has a six-bed combined medical and surgical open intensive care unit (ICU) that is divided into two separate rooms. During the study period, the average length of a hospital stay was 3.2 days (during 2009) to 3.4 days (during 2011). A case patient was defined as a person whose clinical sample yielded carbapenem-resistant K. pneumoniae during the study period. Nosocomial infections were defined by standard Centers for Disease Control and Prevention definitions (13).
Bacterial identification and susceptibility testing.
Identification and initial antibiotic susceptibility testing of isolated strains were performed with the Microscan WalkAway system (Siemens Healthcare Diagnostics, West Sacramento, CA). MICs for several antimicrobials were determined by Etest (bioMérieux, Marcy l'Etoile, France) following CLSI guidelines and interpretative criteria (9). For tigecycline, the U.S. FDA-recommended breakpoints were used (susceptible, ≤2 μg/ml; resistant, ≥8 μg/ml), and for colistin, the CLSI-recommended breakpoints for Acinetobacter spp. were used (susceptible, ≤2 μg/ml; resistant, ≥4 μg/ml).
Phenotypic testing.
Phenotypic tests employing disks of meropenem without and with phenylboronic acid (PBA), EDTA, or both PBA and EDTA were used for the detection of carbapenemase genes (30). Additionally, a modified CLSI test with the addition of PBA was used to detect the coproduction of extended-spectrum β-lactamase (ESBL) genes (31).
PCR testing and nucleotide sequencing.
K. pneumoniae isolates were screened for β-lactamase genes by PCR amplification using a panel of primers for the detection of MBLs, KPCs (30), OXA-48 (22), plasmid-mediated AmpC-encoding genes in a single PCR for each gene (21), and ESBLs, including SHV, TEM, CTX-M, and IBC/GES enzymes (32). DNA was extracted from overnight cultures using the Wizard DNA extraction kit (Promega, Madison, WI). The PCR products which were subjected to direct sequencing were purified using ExoSAP-IT reagent (USB Corporation, Cleveland, OH) and used as templates for sequencing on both strands with an ABI Prism 377 DNA sequencer (Applied Biosystems, Foster City, CA).
Molecular typing.
In order to determine the clonal relationships of the K. pneumoniae isolates, pulsed-field gel electrophoresis (PFGE) of the XbaI-digested genomic DNA was performed using a CHEF-DRIII system (Bio-Rad, Hemel Hempstead, United Kingdom), with a running time of 21 h and pulse times ranging from 3 to 40 s. The PFGE patterns were compared visually according to previously described criteria (28).
Infection control measures during 2009.
During the first year of the study, the following actions were undertaken. (i) Infections caused by carbapenemase-producing pathogens were recorded after phenotypic detection was completed. (ii) A member of the infection control team was responsible for recommending infection control measures, including the use of disposable gloves and gowns and hand washing before and after contact with an infected patient. (iii) In some instances, patients infected with carbapenemase-producing pathogens were cohorted. The medical records of individuals diagnosed with carbapenem-resistant K. pneumoniae infection were reviewed regarding their current and previous hospitalizations. Infections due to carbapenem-resistant K. pneumoniae diagnosed within 48 h of hospital admission were characterized as community onset infections. Community onset infections in patients who had been hospitalized in the preceding 3 months for more than 48 h in hospital facilities or nursing homes were considered health care associated. Finally, infections which developed 48 h following hospital admission were characterized as hospital-acquired infections.
Additional infection control measures during 2010.
After the fourth quarter of 2009, following the rise of infections due to KPC producers, specific infection control interventions were started in January 2010. The measures included the following. (i) The medical and nursing staff was notified immediately after the phenotypic detection of a new case was completed. (ii) Infected patients were isolated or cohorted by transfer to a separate medical or surgical ward or in the case of ICU patients by gathering those infected at one end of the unit. It should be noted that patient isolation in single rooms was not possible in all cases because of their prolonged stay and the limited number of single rooms available. (iii) Infection control measures were reinforced specifically by implementing both the use of disposable gloves and gowns and hand washing with either an antiseptic soap or an alcohol-based hand rub before and after contact with an infected patient. (iv) A continuous program of hand hygiene was promoted, mainly in the ICU, which also included not only monitoring of the compliance of health care workers with hand washing but also providing feedback regarding their performance. (v) The beds of those infected were distinctly labeled. (vi) Relatives and caregivers were informed regarding appropriate infection control measures, hand hygiene, and the possible negative impact of a KPC infection on a patient's outcome. (vii) Patient transfers were limited. (viii) All environmental surfaces and items related to infected patients were cleaned with quaternary ammonium compounds. (ix) Postdischarge terminal cleaning and decontamination were done with appropriate disinfectants such as sodium hypochlorite solutions and sodium dichloroisocyanurate tablets for inanimate surfaces.
Additional infection control measures during 2011.
By the end of 2010, three more single rooms were constructed in the ICU area. This structure made possible the isolation or separate accumulation of at least four ICU patients during 2011. Furthermore, in the last year of the study, the number of dedicated nurses in the ICU increased and specific nurses were assigned solely to the infected patients. The program for hand hygiene promotion was strengthened further both in the ICU and in the wards responsible for hospitalizing infected patients. Finally, the infection control nurse was charged with verifying on a regular basis that all necessary equipment was readily available and used properly and checking the screening culture of newly admitted patients.
Bacteriological studies for infection control purposes.
During the whole period of the study, environmental specimens were obtained from inanimate surfaces, personalized medical devices, and wet surroundings (sinks and baths). This screening was performed in the ICU and the hospital wards whenever two or more cases were identified.
Active surveillance was implemented on a regular basis only during the third year of the study (January to December 2011). Beginning in January 2011, rectal surveillance swabs were obtained from patients on admission to the ICU. The samples were collected using premoistened swabs and plated onto MacConkey agar plates supplemented with meropenem at 1 μg/ml. All Gram-negative colonies growing after 24 h of incubation at 37°C were identified and checked for resistance to carbapenems and possible production of a carbapenemase. This initial phenotypic testing was further verified by molecular techniques.
Data collection and statistical analysis.
The association between the three different periods of infection control interventions and the incidence of new cases per year was assessed with a nonparametric test (Fisher's mid-P exact test) using the statistical software STATA, version 12, 2011. A P value of <0.05 was considered to indicate a statistically significant difference.
RESULTS
Patients and carbapenem-resistant clinical isolates.
During the study period, 84 imipenem- and/or meropenem-resistant K. pneumoniae isolates were recovered from clinical samples from 73 distinct hospitalized patients. Thirty-two (43.8%) of the patients were hospitalized in the ICU, 30 (41.1%) were in medical wards, and 11 (15.1%) were in surgical wards (Fig. 1). The most common type of sample from which these pathogens were isolated was urine (50%), followed by bronchial secretions (17.8%), blood samples (14.3%), pus (13.1%), and intravenous catheters (4.8%). The Etest method was used to confirm MICs of imipenem, meropenem, and ertapenem. All 84 K. pneumoniae isolates exhibited resistance to imipenem and/or meropenem. More specifically, imipenem MICs ranged from 4 to >32 μg/ml while meropenem MICs ranged from 2 to >32 μg/ml and all isolates were highly resistant to ertapenem with MICs of >32 μg/ml. Furthermore, they were resistant to all β-lactams, including β-lactam–β-lactamase inhibitor combinations and aztreonam, amikacin, trimethoprim-sulfamethoxazole, and ciprofloxacin. Antimicrobial alternatives to carbapenems that demonstrated in vitro activity included gentamicin, colistin, and tigecycline, with 74 (88.1%), 76 (90.5%), and 81 (96.4%) of the isolates being susceptible, respectively.
Fig 1.
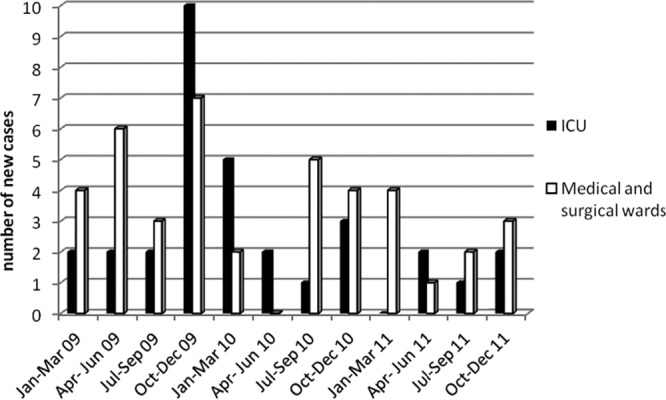
Distribution of new clinical cases due to carbapenemase-producing K. pneumoniae in the ICU and medical and surgical wards.
Phenotyping and molecular testing.
Phenotypic and molecular testing showed that all 84 carbapenem-resistant isolates produced a KPC- and/or an MBL-type carbapenemase. It should be noted that in all cases PCR testing confirmed the results of the phenotypic assays. One representative isolate per patient (a total of 73 isolates) was further tested. PCR and subsequent sequencing analyses revealed that 52 of the patients were infected with KPC-2 producers, 12 were infected with VIM-1 producers, and 9 were infected with isolates producing both KPC-2 and VIM-1 enzymes (Table 1). Phenotypic and molecular testing revealed the coproduction of an ESBL by 36 of the isolates harboring the gene for KPC, in 8 of those harboring the gene for VIM, and in 3 of those that produced both carbapenemases. Plasmidic genes for AmpC were not detected in any of the isolates. The detailed ESBL contents of the clinical isolates are presented in Table 2.
Table 1.
Incidences of imported and hospital-acquired carbapenemase-producing K. pneumoniae cases and carbapenemase gene distributions from 2009 to 2011
| Yr (no. of cases)a | No. of cases/1,000 patient days | No. of imported, hospital acquired cases | No. of imported, hospital-acquired cases/1,000 patient days | No. (%) with blaKPC-2 isolatesb | No. (%) with blaKPC-2 blaVIM-1 isolatesc | No. (%) with blaVIM-1 isolatesd |
|---|---|---|---|---|---|---|
| 2009 (36) | 0.52 | 11, 25 | 0.16, 0.36 | 23 (63.9) | 5 (13.9) | 8 (22.2) |
| 2010 (22) | 0.32 | 9, 13 | 0.13, 0.19 | 16 (72.7) | 4 (18.2) | 2 (9.1) |
| 2011 (15) | 0.21 | 8, 7 | 0.11, 0.1 | 13 (86.7) | 0 | 2 (13.3) |
There were a total of 73 cases.
There were a total of 52 blaKPC-2 cases.
There were a total of 9 blaKPC-2 blaVIM-1 cases.
There were a total of 12 blaVIM-1 cases.
Table 2.
ESBL content and PFGE clonal types of 73 clinical, 11 active surveillance, and 3 environmental carbapenemase-producing K. pneumoniae isolates
| Carbapenemase-producing K. pneumoniae isolate type and source (no. of isolates)a | No. of isolates with: |
PFGE type(s) | |||
|---|---|---|---|---|---|
| SHV-5 | SHV-12 | CTX-M-15 | Any ESBL | ||
| blaKPC-2 (65) | |||||
| Clinical (52) | 34 | 2 | 36 | Ia, Ib, Ic, IIa, IIb, III | |
| Active surveillance (11) | 8 | 8 | Ia, Ib, IIa | ||
| Environmental (2) | Ic | ||||
| blaKPC-2 blaVIM-1 (10) | |||||
| Clinical (9) | 2 | 1 | 3 | IV, Va, Vb, VI | |
| Environmental (1) | Vb | ||||
| blaVIM-1, clinical (12) | 5 | 3 | 8 | VIIa, VIIb, VIIIa, VIIIb | |
There were a total of 87 carbapenemase-producing K. pneumoniae isolates.
Molecular typing.
PFGE analysis of the representative isolates revealed that the 52 KPC-producing K. pneumoniae isolates belonged to three clonal types (I to III), of which one contained three distinct subtypes and another contained two (Table 2; Fig. 2). PFGE type I prevailed during the first year of the study, and PFGE type II prevailed during the subsequent years. The nine isolates that produced both KPC and VIM carbapenemases also belonged to three distinct PFGE clonal types (IV to VI), one of which contained two subtypes. The 12 VIM-producing K. pneumoniae isolates formed two distinct pulsotypes (VII and VIII) with two subtypes each.
Fig 2.
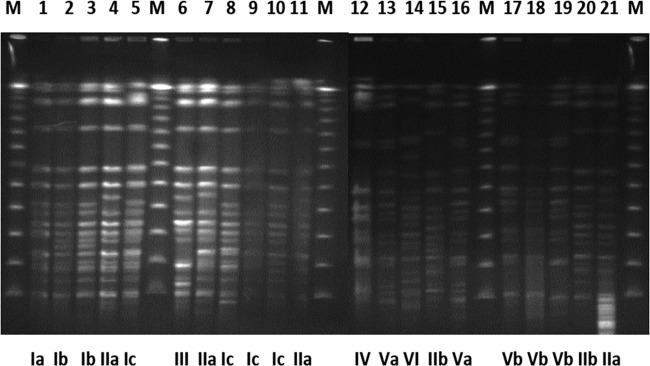
PFGE clonal types and subtypes detected among carbapenemase-producing K. pneumoniae isolates. Lanes 1 to 11, 15, 20, and 21, representative isolates from KPC-producing K. pneumoniae clinical cases; lanes 12 to 14 and 16 to 18, representative isolates from KPC- and VIM-producing K. pneumonia clinical cases; lane 19, KPC- and VIM-producing K. pneumoniae environmental isolate; lanes M, molecular size markers.
Hospitalization history of infected patients.
The clinical specimens that harbored carbapenemase-producing isolates were collected from 28 of the 73 patients within 48 h after their admission, and therefore the respective infections were characterized as community onset. The remaining 45 patient cases were considered hospital acquired. It should be noted that community onset cases were attributed to K. pneumoniae isolates producing either the KPC-2 carbapenemase (n = 23) or both the KPC-2 and VIM-1 carbapenemases (n = 5). Medical histories revealed that patients with community onset infections had been hospitalized over the preceding 3 months in various tertiary-care hospitals in Greece. Therefore, all of these cases were considered imported health care associated. It is also worth mentioning that based on molecular typing, carbapenemase gene content, and the proximity of the patients, 23 of the 45 hospital-acquired cases were epidemiologically linked to imported cases and were considered importation associated.
Comparison of new clinical cases during the 3 years of the study.
Almost half of the carbapenemase-producing clinical isolates (36; 49.3%) were identified during the first year of the study (January to December 2009), when no specific infection control measures had been undertaken. It should be noted that during the fourth quarter of 2009, as many as 10 cases were detected in the ICU (Fig. 1). The number of clinical cases due to carbapenemase-producing K. pneumoniae decreased considerably during the second year of the study (22 cases; 30.1%) and even more in the third year of the study (15 cases; 20.5%) (Table 1).
The incidence of new clinical cases per 1,000 patient days per quarter during the 3 years of the study is presented in Fig. 3. Compared with the incidence of new cases in the first year of the study (preintervention period), there was a considerable decline in the second year (first year of the postintervention period) but not to a significant level (0.52 case/1,000 patient days versus 0.32 case/1,000 patient days, respectively; P = 0.075). After the implementation of the additional infection control measures in the third year of the study, the number of new cases declined to 0.21/1,000 patient days, which differs significantly from the first year of the study (P = 0.0028). Furthermore, the incidence of new cases (0.27 case/1,000 patient days) in the entire postintervention period (January 2010 to December 2011) was significantly lower than that in the preintervention period (P = 0.0051).
Fig 3.
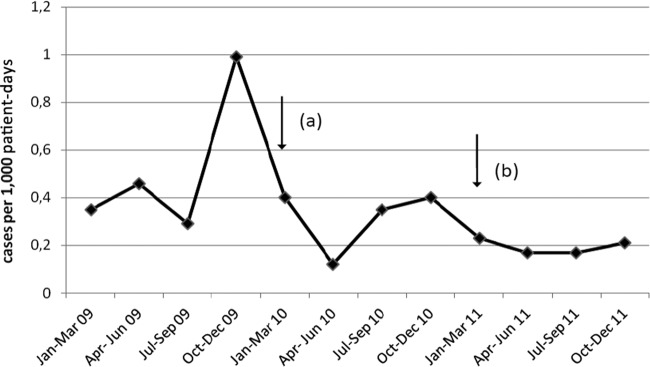
Incidence of new clinical cases due to carbapenemase-producing K. pneumoniae per 1,000 patient days per quarter during the 3 years of this study. Arrows a and b indicate the starting points of the additional infection control intervention measures undertaken in January 2010 and January 2011, respectively.
It is of note that while imported KPC-producing cases showed an even distribution over the 3 years of the study, there was an evident decline in hospital-acquired cases. More specifically, hospital-acquired cases declined from 25 in 2009 (0.36 case/1,000 patient days) to 13 in 2010 (0.19 case/1,000 patient days; P = 0.058) and finally 7 in 2011 (0.1 case/1,000 patient days; P = 0.0012), when active surveillance and other infection control measures were undertaken. It should also be stressed that the incidence of hospital-acquired clinical cases (0.15 case/1,000 patient days) in the entire postintervention period (2010 and 2011) differs significantly from that in the preintervention period (P = 0.0025).
Environmental sampling and active surveillance.
Environmental sampling detected only three carbapenem-resistant isolates during the ICU outbreak in the last quarter of 2009. Two isolates were recovered from ventilator samples, and one was from a bed sample. The KPC-2 enzyme was detected in two of these isolates, while the remaining one harbored both carbapenemases. PFGE analysis revealed that these environmental K. pneumoniae isolates were classified into clonal subtypes that were identified in the clinical isolates (Table 2; Fig. 2).
During the third year of the study, active surveillance of 97 (75.8%) of the 128 patients was performed within 24 h after their admission to the ICU. As many as 11 (11.3%) of the patients screened were found to be colonized by carbapenem-resistant K. pneumoniae isolates. Molecular testing showed that all of the isolates produced the KPC-2 enzyme and eight also produced an ESBL; they shared clonal subtypes which were identified among clinical isolates (Table 2). In all cases, colonized patients were transferred to single ICU rooms and rigorous infection control measures were undertaken. Three of the 11 patients with positive cultures developed clinical infections due to KPC-2-producing K. pneumoniae within 4 to 16 days after their initial screening. In these cases, the surveillance culture led to earlier recognition of the clinical infection due to a KPC-producing pathogen.
DISCUSSION
The emergence and spread of Enterobacteriaceae harboring carbapenemases have brought about several problems regarding infection control and treatment (12). Carbapenemase genes are typically transposon- and/or integron-carried determinants that can easily disseminate among bacterial pathogens in the hospital environment (19). It has, in fact, been proposed that, given the ease of transfer and acquisition of these genes, outbreaks must be controlled early on in order to avoid hospital dissemination (3, 6, 12). Indeed, surveys in regions of endemicity, such as Israel, Italy, and the northeastern United States, have reported successful control management of infections caused by KPC producers (1, 2, 4, 8, 17, 26). In the present prospective study, we documented the effectiveness of containment strategies in combating the secondary transmission of imported KPC clones from other Greek tertiary-care facilities.
Greece is a country where KPC-producing members of the family Enterobacteriaceae have recently become endemic (11, 23, 27). Measures on a national level have already been proposed and implemented in some hospital facilities. In the present survey, previously hospitalized and/or colonized patients seem to represent the main route of introduction of carbapenemase genes into our hospital setting. The fact that several patients were admitted with community onset infections caused by different KPC-producing or KPC- and VIM-producing K. pneumoniae clonal types/subtypes, along with the observation that all of them had a recent history of hospitalization in a tertiary-care facility, suggests that the KPC clones detected in our hospital were frequently introduced from other hospital environments. This hypothesis was further supported by the different broad-spectrum β-lactamase profiles detected among these isolates.
The high incidence of carbapenemase-possessing pathogens and especially of KPC producers, in the first year of the study, was possibly due to the insufficiency of the infection control measures, which in several instances did not adhere to specific requirements for the control of infections with carbapenemase-producing pathogens in acute-care facilities (7). Thus, the imported clonal strains were successfully disseminated in our hospital environment and were responsible for the KPC outbreak in our ICU during the fourth quarter of 2009. During this period, the secondary transmission of imported hospital-associated KPC-producing strains was considered high. It should also be mentioned that microbiological screening of the hospital environment revealed that the immediate surroundings of the infected patients played a role in the dissemination of KPC clones in our unit.
However, during the entire postintervention period (January 2010 to December 2011), the reinforcement of infection control measures, based on the guidance for carbapenemase-producing Enterobacteriaceae prevention (7) with early warning, promotion of hand hygiene, and immediate initiation of contact precautions, led to a considerable reduction of hospital-acquired carbapenemase producers. Our findings also suggest that a significant containment of carbapenem-resistant K. pneumoniae strains was made possible especially during the third year of the survey with the implementation of a comprehensive infection control intervention program which included active surveillance, carrier isolation/cohorting, and dedicated staff for those infected. It should be noted that patients' isolation in single rooms was possible in 8 (36.4%) of 22 cases during 2010 and in as many as 11 (73.3%) of 15 cases during 2011. The hand hygiene compliance of health care workers was also increased during the postintervention period. More specifically, compliance improved from 22 to 43% in hospital wards while it improved from 27 to 56% in the ICU. In addition, our observations reinforce the necessity of employing rectal surveillance cultures in order to restrain the dissemination of KPC-producing clones (2, 5, 8, 10, 15). Although rectal swab screening of all of our new ICU admissions was not implemented, this practice was found to be very useful, since it made possible the early recognition of KPC clonal strains likely to be introduced into our hospital, especially in the cases of patients with previous hospitalizations.
Highly sensitive tests for the rapid detection of carbapenemases, a fast diagnostic turnaround time, and timely communication of laboratory results are necessary for the implementation of an effective infection control program (3, 7, 12). In our surveillance, accurate and early phenotypic detection of carbapenemase-producing pathogens was proven to be vital in guiding toward the appropriate therapy and in enforcing infection control measures. Phenotypic testing can also give information regarding both the type of carbapenemase involved and the possibility of ESBL coproduction (30, 31). This preliminary testing allowed the accomplishment of early and appropriate cohorting that, along with contact precautions, restrained the dissemination of imported carbapenem-resistant clones in our facilities. It should also be noted that sporadic KPC-producing strains of the same clonal type were recovered from environmental ICU samples during the first year of the survey. This underscored the need for meticulous environmental cleaning and disinfection following infected patients' discharge.
In conclusion, the present study emphasizes that in regions where KPC clones are endemic, the cooperation of the clinical microbiology laboratory, the infection control team, and the medical and nursing staff is still vital in order to design and implement appropriate infection control measures for carbapenemase-producing pathogens. The use of active surveillance as part of this multifactorial intervention may also enhance strategies to decrease the rates of secondary transmission of imported genes. Other interventions, such as shortening of the average length of stay, might further augment infection control actions. It should also be of interest to examine whether our interventions also change the prevalence of other, less common, multidrug-resistant pathogens.
Footnotes
Published ahead of print 30 May 2012
REFERENCES
- 1. Agodi A, et al. 2011. Containment of an outbreak of KPC-3-producing Klebsiella pneumoniae in Italy. J. Clin. Microbiol. 49:3986–3989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ben-David D, et al. 2010. Potential role of active surveillance in the control of a hospital-wide outbreak of carbapenem-resistant Klebsiella pneumoniae infection. Infect. Control Hosp. Epidemiol. 31:620–626 [DOI] [PubMed] [Google Scholar]
- 3. Bilavsky E, Schwaber MJ, Carmeli Y. 2010. How to stem the tide of carbapenemase-producing Enterobacteriaceae: proactive versus reactive strategies. Curr. Opin. Infect. Dis. 23:327–331 [DOI] [PubMed] [Google Scholar]
- 4. Borer A, et al. 2011. A multifaceted intervention strategy for eradication of a hospital-wide outbreak caused by carbapenem-resistant Klebsiella pneumoniae in southern Israel. Infect. Control Hosp. Epidemiol. 32:1158–1165 [DOI] [PubMed] [Google Scholar]
- 5. Calfee D, Jenkins SG. 2008. Use of active surveillance cultures to detect asymptomatic colonization with carbapenem-resistant Klebsiella pneumoniae in intensive care unit patients. Infect. Control Hosp. Epidemiol. 29:966–968 [DOI] [PubMed] [Google Scholar]
- 6. Carmeli Y, et al. 2010. Controlling the spread of carbapenemase-producing Gram-negatives: therapeutic approach and infection control. Clin. Microbiol. Infect. 16:102–111 [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention 2009. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR Morb. Mortal. Wkly. Rep. 58:256–260 [PubMed] [Google Scholar]
- 8. Ciobotaro P, Oved M, Nadir E, Bardenstein R, Zimhony O. 2011. An effective intervention to limit the spread of an epidemic carbapenem-resistant Klebsiella pneumoniae strain in an acute care setting: from theory to practice. Am. J. Infect. Control 39:671–677 [DOI] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing. Twentieth informational supplement update. Document M100-S20 U. CLSI, Wayne, PA [Google Scholar]
- 10. Cohen MJ, et al. 2011. Institutional control measures to curtail the epidemic spread of carbapenem-resistant Klebsiella pneumoniae: a 4-year perspective. Infect. Control Hosp. Epidemiol. 32:673–678 [DOI] [PubMed] [Google Scholar]
- 11. Giakkoupi P, et al. 2011. An update of the evolving epidemic of blaKPC-2-carrying Klebsiella pneumoniae in Greece (2009-10). J. Antimicrob. Chemother. 66:1510–1513 [DOI] [PubMed] [Google Scholar]
- 12. Grundmann H, et al. 2010. Carbapenem-non-susceptible Enterobacteriaceae in Europe: conclusions from a meeting of national experts. Euro Surveill. 15(46).pii:19711 [DOI] [PubMed] [Google Scholar]
- 13. Horan TC, Andrus M, Dudeck MA. 2008. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36:309–332 [DOI] [PubMed] [Google Scholar]
- 14. Kitchel B, et al. 2009. Molecular epidemiology of KPC-producing Klebsiella pneumoniae in the United States: clonal expansion of MLST sequence type 258. Antimicrob. Agents Chemother. 53:3365–3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kochar S, et al. 2009. Success of an infection control program to reduce the spread of carbapenem-resistant Klebsiella pneumoniae. Infect. Control Hosp. Epidemiol. 30:447–452 [DOI] [PubMed] [Google Scholar]
- 16. Marchaim D, Navon-Venezia S, Schwaber MJ, Carmeli Y. 2008. Isolation of imipenem-resistant Enterobacter species: emergence of KPC-2 carbapenemase, molecular characterization, epidemiology, and outcomes. Antimicrob. Agents Chemother. 52:1413–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Munoz-Price LS, et al. 2010. Successful control of an outbreak of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae at a long-term acute care hospital. Infect. Control Hosp. Epidemiol. 31:341–347 [DOI] [PubMed] [Google Scholar]
- 18. Nordmann P, Cuzon G, Naas T. 2009. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9:228–236 [DOI] [PubMed] [Google Scholar]
- 19. Nordmann P, Naas T, Poirel L. 2011. Global spread of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 17:1791–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect. Control Hosp. Epidemiol. 29:1099–1106 [DOI] [PubMed] [Google Scholar]
- 21. Pérez-Pérez FJ, Hanson ND. 2002. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J. Clin. Microbiol. 40:2153–2162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poirel L, Héritier C, Tolün V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 48:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pournaras S, et al. 2009. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J. Antimicrob. Chemother. 64:348–352 [DOI] [PubMed] [Google Scholar]
- 24. Pournaras S, et al. 2010. Detection of the new metallo-β-lactamase VIM-19 along with KPC-2, CMY-2 and CTX-M-15 in Klebsiella pneumoniae. J. Antimicrob. Chemother. 65:1604–1607 [DOI] [PubMed] [Google Scholar]
- 25. Schwaber MJ, et al. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob. Agents Chemother. 52:1028–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwaber MJ, et al. 2011. Containment of a country-wide outbreak of carbapenem-resistant Klebsiella pneumoniae in Israeli hospitals via a nationally implemented intervention. Clin. Infect. Dis. 52:848–855 [DOI] [PubMed] [Google Scholar]
- 27. Souli M, et al. 2010. An outbreak of infection due to β-lactamase Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae in a Greek university hospital: molecular characterization, epidemiology, and outcomes. Clin. Infect. Dis. 50:364–373 [DOI] [PubMed] [Google Scholar]
- 28. Tenover FC, et al. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tsakris A, et al. 2008. First occurrence of KPC-2-possessing Klebsiella pneumoniae in a Greek hospital and recommendation for detection with boronic acid disc tests. J. Antimicrob. Chemother. 62:1257–1260 [DOI] [PubMed] [Google Scholar]
- 30. Tsakris A, et al. 2010. A simple phenotypic method for the differentiation of metallo-β-lactamases and class A KPC carbapenemases in Enterobacteriaceae clinical isolates. J. Antimicrob. Chemother. 65:1664–1671 [DOI] [PubMed] [Google Scholar]
- 31. Tsakris A, et al. 2009. Use of boronic acid disk tests to detect extended-spectrum β-lactamases in clinical isolates of KPC carbapenemase-possessing Enterobacteriaceae. J. Clin. Microbiol. 47:3420–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tzelepi E, et al. 2003. Extended-spectrum β-lactamase types in Klebsiella pneumoniae and Escherichia coli in two Greek hospitals. Int. J. Antimicrob. Agents 21:285–288 [DOI] [PubMed] [Google Scholar]
- 33. Zarkotou O, et al. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin. Microbiol. Infect. 17:1798–1803 [DOI] [PubMed] [Google Scholar]


