Abstract
Our aim was to determine Trichomonas vaginalis prevalence using the Aptima Trichomonas vaginalis assay (ATV; Gen-Probe) and the prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae coinfections in U.S. women undergoing screening for C. trachomatis/N. gonorrhoeae. Discarded urogenital samples from 7,593 women (18 to 89 years old) undergoing C. trachomatis/N. gonorrhoeae screening using the Aptima Combo 2 assay (Gen-Probe) in various clinical settings were tested with ATV. Overall, T. vaginalis, C. trachomatis, and N. gonorrhoeae prevalences were 8.7%, 6.7%, and 1.7%, respectively. T. vaginalis was more prevalent than C. trachomatis or N. gonorrhoeae in all age groups except the 18- to 19-year-old group. The highest T. vaginalis prevalence was in women ≥40 years old (>11%), while the highest C. trachomatis prevalence (9.2%) and N. gonorrhoeae prevalence (2.2%) were in women <30 years old. Coinfection prevalences were 1.3% for C. trachomatis/T. vaginalis, 0.61% for C. trachomatis/N. gonorrhoeae and N. gonorrhoeae/T. vaginalis, and 0.24% for C. trachomatis/N. gonorrhoeae/T. vaginalis and highest in women <30 years old. T. vaginalis prevalence differed by race/ethnicity, with the highest prevalence in black women (20.2%). T. vaginalis prevalence ranged from 5.4% in family planning clinics to 22.3% in jails. Multivariate analysis determined that ages of ≥40 years, black race, and patient locations were significantly associated with T. vaginalis infection. T. vaginalis is the most common sexually transmitted infection (STI) in women of >40 years, while C. trachomatis and N. gonorrhoeae prevalence is lowest in that age group. Higher T. vaginalis prevalence in women of >40 years is probably attributed to the reason for testing, i.e., symptomatic status versus routine screening in younger women. Coinfections were relatively low. High T. vaginalis prevalence in all age groups suggests that women screened for C. trachomatis/N. gonorrhoeae, whether asymptomatic or symptomatic, should be screened for T. vaginalis.
INTRODUCTION
Trichomoniasis, caused by the protozoan Trichomonas vaginalis, is a common sexually transmitted infection (STI) affecting men and women, with an estimated 7.4 million cases annually in the United States (2, 6, 35). Approximately 50 to 60% of T. vaginalis infections in women are asymptomatic (1). Available effective treatment makes T. vaginalis one of the most curable STIs (35). Untreated T. vaginalis infections may result in long-term sequelae, such as pelvic inflammatory disease, preterm births, or low-birth-weight infants (7, 8). Moreover, T. vaginalis infection has been shown to increase the risk of HIV sexual transmission (20, 22, 31). Thus, detection of T. vaginalis is key for treatment, reducing transmission, and preventing associated negative health outcomes.
Currently, the gold standard for detection of T. vaginalis infection in women is culture of vaginal specimens. However, culture is only moderately sensitive (∼75%) compared to nucleic acid amplification tests (NAATs), is costly, and takes 3 to 7 days to provide results, thus making it unsuitable for routine clinical use (18, 25). More-rapid techniques include microscopy (“wet mount”) of vaginal fluid, the most commonly used test in the clinic, and antigen detection (OSOM Trichomonas rapid test; Genzyme Diagnostics). However, sensitivity is low (∼50%) for microscopy and only moderate (82%) for antigen tests (18, 25). These tests also show poorer sensitivity in asymptomatic populations. Furthermore, by microscopy, it is difficult to differentiate T. vaginalis from Pentatrichomonas hominis, a nonpathogenic gastrointestinal commensal flagellate that can contaminate samples during collection. Another diagnostic option is the Affirm VP III DNA probe test (Becton, Dickinson, San Jose, CA), which has moderate sensitivity (64 to 80%) compared to wet mount and/or culture (3, 4). PCR has moderately high sensitivity for T. vaginalis detection in vaginal fluids (80% to 95%), but there is no U.S. Food and Drug Administration (FDA)-approved PCR test for diagnosis of T. vaginalis infection (14, 21, 25, 26, 34). The only FDA-cleared NAAT for T. vaginalis is the Aptima Trichomonas vaginalis assay (ATV; Gen-Probe Inc., San Diego, CA). Sample types include urine, clinician-collected vaginal swabs (CVS), endocervical swabs (ES), and PreservCyt solution (Hologic Incorporated, Bedford, MA) for liquid-based cytology (PCyt). A multicenter study that determined ATV performance for FDA clearance demonstrated that assay sensitivity ranged from 95.2% for urine to 100% for CVS, ES, and PCyt samples (28). ATV was highly specific for T. vaginalis (≥98.9%) (28). Additional U.S. studies demonstrated that ATV has the highest sensitivity (>95%) in vaginal fluid of the available assays, including PCR, and has similarly high sensitivity in other sample types (urine, ES, and PCyt) (3, 13, 16, 18, 25). Andrea and Chapin demonstrated that the sensitivity of Affirm was only 64% in a comparison with ATV (3).
The 2001 to 2004 National Health and Nutrition Examination Surveys (NHANES) used PCR testing to determine the first national T. vaginalis infection prevalence (∼3.2%) in American women 14 to 49 years old (1, 29). T. vaginalis infection was more prevalent than Chlamydia trachomatis (2.2%) and Neisseria gonorrhoeae (0.24%) infections combined among women 14 to 39 years old (9). The National Longitudinal Study of Adolescent Health, a prospective cohort study, used PCR testing and determined that the estimated T. vaginalis prevalence in young women was 2.8% (23). Using 1,525 home-collected mailed vaginal swabs and ATV, a recent report detected a T. vaginalis prevalence of 10% (11). The 7.4 million incident T. vaginalis infections per year makes T. vaginalis the most prevalent nonviral and treatable STI in the United States (2, 33). Despite high prevalence, T. vaginalis infections are currently not required to be reported to the U.S. Centers for Disease Control and Prevention (CDC; Atlanta, GA).
The goal of this study was to determine T. vaginalis prevalence by using the highly sensitive ATV assay in a large sample of women aged 18 or older that were undergoing C. trachomatis/N. gonorrhoeae screening in a wide geographical area and by comparing T. vaginalis prevalence to C. trachomatis and N. gonorrhoeae prevalence in the same samples.
(This work has been presented in part as an oral presentation at the International Society for Sexually Transmitted Disease Research [ISSTDR] Meeting, Quebec, Canada, July 2011.)
MATERIALS AND METHODS
Overall study design.
Discarded, deidentified clinical specimens, collected July through November 2010 from women for C. trachomatis/N. gonorrhoeae testing with the Aptima Combo 2 assay (AC2; Gen-Probe Inc., San Diego, CA) on the Tigris DTS instrumentation system (Tigris; Gen-Probe) were used. AC2 is a NAAT that utilizes target capture specimen processing, transcription-mediated amplification (TMA), chemiluminescent probe hybridization, and the automated Tigris system to detect C. trachomatis and N. gonorrhoeae ribosomal RNAs (rRNAs) in urogenital samples. Samples from each participating laboratory were tested with the research-use-only ATV kit on the Tigris system. Each laboratory reported AC2 and ATV results and available deidentified demographic data to a centralized database. Prevalence for T. vaginalis, C. trachomatis, and N. gonorrhoeae and coinfection rates were determined.
Study population and sites.
The study population included women 18 to 89 years old from 21 states who were with or without symptoms of STIs. Due to the use of discarded, deidentified samples, information on symptoms, sexual history, or STI history was not available. Therefore, based on current CDC C. trachomatis/N. gonorrhoeae screening recommendations (5, 35), samples collected from younger patients (<26 years) were probably more heavily weighted for routine screening (asymptomatic) than samples collected from older women which were probably more associated with clinical symptoms. Collection sites included patients treated in a hospital setting (emergency departments [ED] or hospitalized), obstetric/gynecologic (OB/GYN) practices, family planning clinics, family practices, sexually transmitted disease (STD) clinics, or other clinics performing tests for STIs. Eighteen laboratories throughout the United States using AC2 participated. Ethical approval was obtained at each site in accordance with institutional review board policies to release deidentified results and demographic information to Gen-Probe.
Clinical specimens.
A total of 7,593 specimens, including self- or physician-collected vaginal swabs (CVS; n = 791), ES (n = 3,912), urine (n = 1,945), and ES specimens collected in PCyt (PCyt; n = 940), were obtained and tested for C. trachomatis/N. gonorrhoeae and T. vaginalis. Only one specimen per patient, independent of specimen type, was tested. All specimens were placed into the appropriate Aptima transport tubes before being shipped to the testing sites. CVS and ES specimens were added directly into transport buffer tubes upon collection. Urine samples were aliquoted at the collection site into Aptima urine specimen transport tubes, and for PCyt samples, a 1-ml portion was transferred into a specimen transfer kit. Each site collected a minimum of 350 patient specimens. Specimens were stored at 2 to 30°C and tested with ATV within 30 days of collection or up to 60 days after collection if using ES or CVS. This is the current time frame for testing C. trachomatis/N. gonorrhoeae from these sample types for the Aptima Combo 2 assay. In-house data (Gen-Probe, Inc.) have shown no loss of or decrease in ability to detect the T. vaginalis target for the sample types listed under the conditions described for collection and storage that are currently published in the package insert for the Aptima T. vaginalis product.
ATV assay and sample testing.
ATV is a NAAT that utilizes the same technology and instrumentation as AC2 to detect T. vaginalis rRNA in urogenital samples. Consecutive female samples meeting study entry criteria were tested in accordance with ATV instructions on the Tigris instrument.
Data collection and statistical analysis.
ATV results were transmitted electronically to a central database. Associated C. trachomatis/N. gonorrhoeae results and deidentified demographic data (age, sex, race, clinic type, and location [identified by the first 3 digits of the zip code]) were entered into an electronic report form and sent for statistical analysis performed primarily with PC SAS version 9.2 or higher (Cary, NC). For categorical variables, summary statistics included the number of subjects and percentage for each category. For continuous variables, summary statistics included the number of observations and the mean, standard deviation, median, minimum, and maximum values. Age-adjusted odds ratios (ORs) were calculated via a multivariable logistic regression model controlling for age in categories (<20, 20 to 29, 30 to 39, and ≥40 years), whereas multivariable ORs further controlled for collection site, race, and geographical regions determined as Northeast (Delaware, Maryland, Rhode Island, Connecticut, New Jersey, New York, Pennsylvania), Southeast (Louisiana, Florida, Georgia, Kansas, Kentucky, Tennessee, Virginia), Southwest (California, Texas, New Mexico, Arizona, Colorado, Nevada, Utah), and Midwest (Indiana, Michigan, Minnesota, Ohio, Illinois, Missouri, Wisconsin). No patient specimens were collected from the Northwest region.
RESULTS
Overall prevalence of T. vaginalis, C. trachomatis, and N. gonorrhoeae infections.
Overall prevalences of T. vaginalis, C. trachomatis, and N. gonorrhoeae infections were 8.7%, 6.7%, and 1.7%, respectively (Table 1). Rates of coinfection were low (≤1.3%): 1.3% of subjects were coinfected with T. vaginalis and C. trachomatis, 0.61% were coinfected with T. vaginalis and N. gonorrhoeae, 0.61% were coinfected with C. trachomatis and N. gonorrhoeae, and 0.24% of subjects had a triple coinfection (T. vaginalis, C. trachomatis, and N. gonorrhoeae).
Table 1.
Prevalence of T. vaginalis, C. trachomatis, and N. gonorrhoeae infections by age, race, and collection site
| Characteristic or prevalence categorya | n | Prevalence (%)b |
||||||
|---|---|---|---|---|---|---|---|---|
| T. vaginalis | C. trachomatis | N. gonorrhoeae | C. trachomatis/N. gonorrhoeae/T. vaginalis/ | C. trachomatis/T. vaginalis/ | C. trachomatis/N. gonorrhoeae/ | N. gonorrhoeae/T. vaginalis/ | ||
| Overall prevalence (no. infected/total no. of samples) | 7,593 | 8.7 (663/7,593) | 6.7 (508/7,588) | 1.7 (129/7,579) | 0.24 (18/7,577) | 1.3 (97/7,588) | 0.61 (46/7,577) | 0.61 (46/7,579) |
| Mean age (yr) (SD) | 29.82 (10.62) | 23.4 (6.0) | 24.0 (6.9) | 22.00 (2.35) | 23.41 (5.49) | 21.89 (3.78) | 23.15 (5.53) | |
| Median age (yr) (minimum-maximum) | 26.0 (18–82) | 22.0 (18–65) | 22.0 (18–53) | 21.5 (18–26) | 22 (18–46) | 21 (18–37) | 21.5 (18–50) | |
| Age | ||||||||
| ≥18 and <20 | 907 | 8.5 | 14.4 | 3.3 | 0.2 | 2.1 | 1.3 | 0.9 |
| ≥20 and <30 | 3,975 | 8.3 | 8.0 | 2.0 | 0.4 | 1.7 | 0.8 | 0.9 |
| ≥30 and <40 | 1,667 | 7.9 | 2.5 | 0.8 | 0.0 | 0.4 | 0.1 | 0.2 |
| ≥40 | 1,044 | 11.8 | 1.7 | 0.5 | 0.0 | 0.3 | 0.0 | 0.1 |
| Race | ||||||||
| White | 1,668 | 5.7 | 5.7 | 1.6 | 0.1 | 0.6 | 0.4 | 0.5 |
| Black | 1,385 | 20.2 | 12.1 | 4.0 | 0.7 | 3.6 | 1.7 | 1.7 |
| Hispanic/Latino | 718 | 5.0 | 5.9 | 0.7 | 0.0 | 0.6 | 0.0 | 0.1 |
| All others/unknownc | 3,822 | 6.6 | 5.3 | 1.1 | 0.2 | 0.9 | 0.5 | 0.3 |
| Collection site | ||||||||
| Family planning | 784 | 5.4 | 10.4 | 1.5 | 0.0 | 1.4 | 0.5 | 0.1 |
| Hospitald | 493 | 16.6 | 7.3 | 2.2 | 1.0 | 3.0 | 1.0 | 1.6 |
| Family practice, internal medicine | 817 | 6.1 | 7.8 | 1.2 | 0.0 | 0.4 | 0.4 | 0.0 |
| OB/GYN | 2,059 | 7.3 | 4.4 | 0.8 | 0.2 | 0.9 | 0.3 | 0.3 |
| Jail, STD clinic | 1,304 | 16.4 | 10.5 | 3.5 | 0.5 | 2.5 | 1.3 | 1.5 |
| Other/unknown | 2,136 | 5.9 | 4.6 | 1.6 | 0.2 | 0.8 | 0.5 | 0.6 |
SD, standard deviation.
In the calculations of prevalence, the denominator may be less than the number shown for n due to missing or invalid assay data. Values are the prevalence unless otherwise indicated in the stub entry for that row (i.e., mean and median age). Values given in parentheses are identified by the information in parentheses in the stub entry for that row.
“Other” races include American Indian, Native Alaskan, Asian, and Native Hawaiian/Pacific Islander.
Consists of patients seen in an emergency department and/or hospitalized.
Prevalence of T. vaginalis, C. trachomatis, and N. gonorrhoeae infections by age.
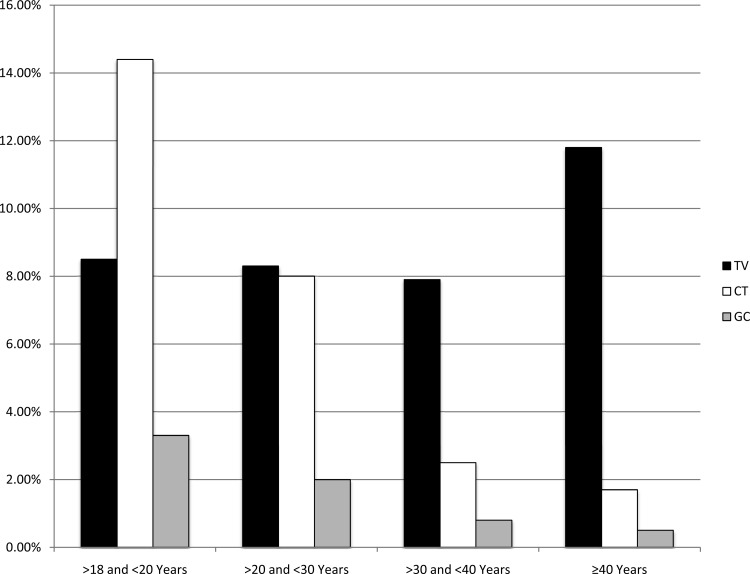
Of the three STIs surveyed, T. vaginalis was the most prevalent in all age groups except the 18- to 19-year-old group, for which C. trachomatis was the most prevalent (C. trachomatis, 14.4%; T. vaginalis, 8.5%; N. gonorrhoeae, 3.3%) (Table 1 and Fig. 1). T. vaginalis prevalence was lowest in women <40 years old (range, 7.9% to 8.5%) and highest in women ≥40 years old (11.3% in women 40 to 49 years old, 13.0% in women ≥50 years old). In contrast, C. trachomatis and N. gonorrhoeae prevalence was lowest in women ≥40 years old (<2%) and highest in women <30 years old (ranges, 8.0% to 14.4% for C. trachomatis and 2.0% to 3.3% for N. gonorrhoeae). Double and triple coinfections were more common in women <30 years old than in women ≥30 years old (Table 1). Univariate and multivariate analyses (Table 2) revealed a significant association between T. vaginalis infections and women aged ≥40 years.
Fig 1.
Prevalence rates by age. TV, Trichomonas vaginalis; CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae. Prevalence rates for women of younger ages (<30 years) are biased toward an asymptomatic population per CDC screening guidelines. Higher prevalence rates in older women are biased toward a symptomatic population.
Table 2.
Univariate and multivariate analyses of risk factors associated with T. vaginalis, C. trachomatis, and N. gonorrhoeae infections
| Risk factor | Indicated values for each infectiona |
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
T. vaginalis |
C. trachomatis |
N. gonorrhoeae |
|||||||
| Prevalence (%) | OR (95% CI) | Multivariate analysis OR estimate (95% CI) | Prevalence (%) | OR (95% CI)b | Multivariate analysis OR estimate (95% CI)b | Prevalence (%) | OR (95% CI)c | Multivariate analysis OR estimate (95% CI)c | |
| Age (yr) | |||||||||
| ≥18 and <20 | 8.49 | 1 | 1 | 14.35 | 1 | 1 | 3.31 | 1 | 1 |
| ≥20 and <30 | 8.33 | 0.98 (0.76–1.27) | 1.03 (0.79–1.36) | 8.03 | 0.52 (0.42–0.65) | 0.53 (0.42–0.66) | 2.04 | 0.61 (0.40–0.93) | 0.66 (0.43–1.02) |
| ≥30 and <40 | 7.92 | 0.93 (0.69–1.24) | 1.03 (0.76–1.40) | 2.52 | 0.15 (0.11–0.22) | 0.17 (0.11–0.24) | 0.78 | 0.23 (0.12–0.44) | 0.27 (0.14–0.52) |
| ≥40 | 11.78 | 1.44 (1.07–1.94) | 1.51 (1.10–2.07) | 1.63 | 0.10 (0.06–0.17) | 0.09 (0.06–0.16) | 0.48 | 0.14 (0.05–0.37) | 0.14 (0.06–0.37) |
| Race | |||||||||
| White | 5.70 | 1 | 1 | 5.70 | 1 | 1 | 1.56 | 1 | 1 |
| Black | 20.22 | 4.15 (3.25–5.31) | 4.04 (3.12–5.22) | 12.08 | 2.34 (1.80–3.06) | 2.63 (1.99–3.49) | 3.98 | 2.67 (1.66–4.29) | 2.66 (1.61–4.41) |
| Hispanic/Latino | 5.01 | 0.86 (0.58–1.27) | 0.88 (0.58–1.32) | 5.85 | 1.18 (0.80–1.72) | 1.29 (0.87–1.94) | 0.70 | 0.49 (0.19–1.29) | 0.60 (0.22–1.64) |
| All others/unknown | 6.59 | 1.15 (0.90–1.46) | 0.92 (0.69–1.23) | 5.34 | 1.07 (0.83–1.38) | 1.54 (1.15–2.06) | 1.13 | 0.80 (0.49–1.31) | 0.71 (0.40–1.27) |
| Clinic type | |||||||||
| Family planning | 5.36 | 1 | 1 | 10.36 | 1 | 1 | 1.54 | 1 | 1 |
| Hospital | 16.63 | 3.53 (2.38–5.21) | 3.50 (2.30–5.32) | 7.30 | 0.68 (0.45–1.03) | 0.65 (0.42–1.00) | 2.24 | 1.47 (0.64–3.35) | 2.00 (0.83–4.82) |
| Family practice, internal medicine | 6.12 | 1.15 (0.76–1.76) | 1.27 (0.80–2.01) | 7.83 | 0.74 (0.52–1.04) | 0.87 (0.60–1.28) | 1.23 | 0.80 (0.34–1.87) | 1.62 (0.65–4.02) |
| OB/GYN | 7.29 | 1.39 (0.98–1.98) | 1.33 (0.90–1.98) | 4.42 | 0.40 (0.29–0.55) | 0.40 (0.28–0.57) | 0.78 | 0.50 (0.24–1.07) | 0.73 (0.32–1.70) |
| Jail, STD clinic | 16.41 | 3.47 (2.46–4.89) | 2.59 (1.77–3.80) | 10.52 | 1.02 (0.76–1.36) | 0.91 (0.65–1.26) | 3.45 | 2.29 (1.21–4.36) | 2.73 (1.32–5.65) |
| Other/unknown | 5.85 | 1.10 (0.77–1.57) | 1.67 (1.12–2.49) | 4.64 | 0.42 (0.31–0.57) | 0.47 (0.33–0.66) | 1.64 | 1.07 (0.55–2.07) | 2.36 (1.12–4.95) |
| Region | |||||||||
| Northeast | 4.30 | 1 | 1 | 4.78 | 1 | 1 | 0.92 | 1 | 1 |
| Southwest | 9.51 | 2.35 (1.83–3.01) | 1.88 (1.40–2.50) | 7.76 | 1.82 (1.42–2.34) | 1.51 (1.13–2.03) | 1.68 | 1.94 (1.13–3.34) | 1.64 (0.88–3.06) |
| Southeast | 14.44 | 3.78 (2.87–4.98) | 6.32 (4.62–8.63) | 7.88 | 1.83 (1.34–2.50) | 2.26 (1.60–3.19) | 2.49 | 2.90 (1.59–5.31) | 6.77 (3.45–13.29) |
| Midwest | 9.48 | 2.35 (1.80–3.05) | 2.00 (1.50–2.65) | 6.64 | 1.39 (1.06–1.83) | 1.16 (0.86–1.56) | 2.20 | 2.38 (1.37–4.13) | 2.33 (1.29–4.22) |
Multivariate analysis odds ratio (OR) estimates were adjusted for age, race, clinic type, and region. CI, confidence interval.
Five observations were deleted due to missing values for the response or explanatory variables.
Fourteen observations were deleted due to missing values for the response or explanatory variables.
Prevalence of T. vaginalis, C. trachomatis, and N. gonorrhoeae infections by race.
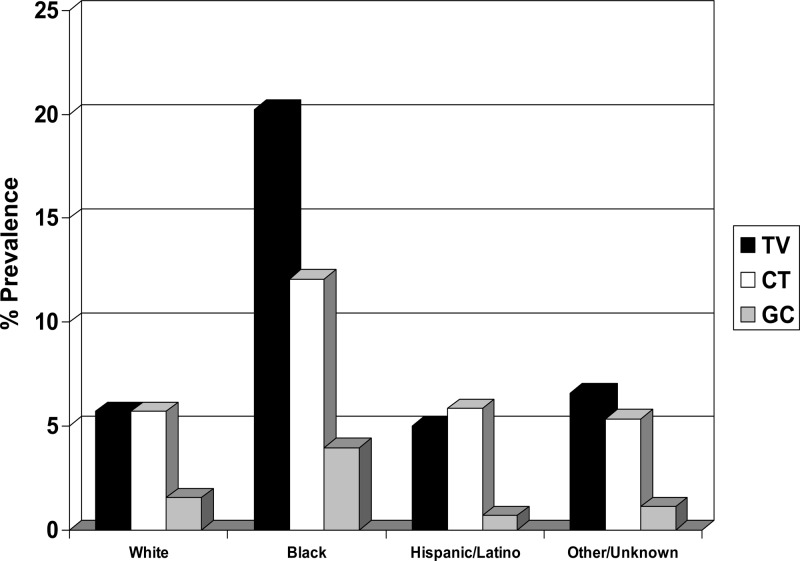
STI prevalence varied greatly by race/ethnicity. For racial groups with >100 subjects, T. vaginalis prevalence was highest for blacks (20.2%), followed by whites (5.7%) and Hispanics/Latinos (5.0%) (Table 1 and Fig. 2). C. trachomatis prevalence was highest for blacks (12.1%), followed by Hispanics/Latinos (5.9%) and whites (5.7%) (Table 1). N. gonorrhoeae prevalence was highest for blacks (4.0%), followed by whites (1.6%) and Hispanics/Latinos (0.7%) (Table 1). Blacks had relatively higher rates of coinfection (T. vaginalis and C. trachomatis, 3.6%; C. trachomatis and N. gonorrhoeae, 1.7%; T. vaginalis and N. gonorrhoeae, 1.7%) than other ethnicities (<1%). Included in the unknown/other category were subsets of ethnic groups, including American Indians/Alaskan Natives (10.5%) and Native Hawaiians/Pacific Islanders (7.1%), with high T. vaginalis prevalence. T. vaginalis, C. trachomatis, and N. gonorrhoeae prevalence rates in the subset of Asian women were 3.8%, 9.9%, and 2.3%, respectively. Univariate and multivariate analyses (Table 2) revealed a significant association between T. vaginalis, C. trachomatis, and N. gonorrhoeae infections and black women.
Fig 2.
Prevalence rates by racial/ethnic group. TV, Trichomonas vaginalis; CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae. The other/unknown group includes American Indians, Alaskan Natives, Native Hawaiians/Pacific Islanders, and Asians.
Prevalence of T. vaginalis, C. trachomatis, and N. gonorrhoeae infections by testing site and geographical location.
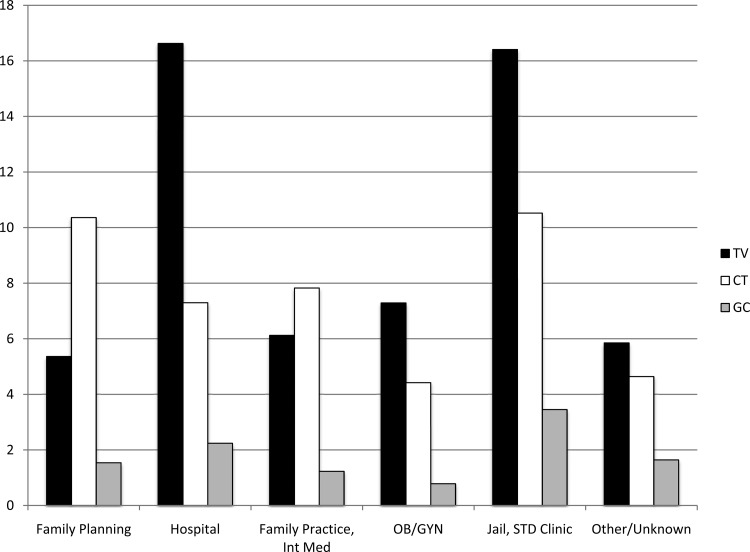
T. vaginalis prevalence varied greatly by sample collection site (Table 1 and Fig. 3) and geographical location (Fig. 4). T. vaginalis prevalence was highest among hospital-associated patients (16.6%) and jail inmates/STD clinic patients (16.4%), followed by OB/GYN practices (7.3%) and family practice/internal medicine practices (6.1%), and lowest in family planning centers (5.4%). C. trachomatis prevalence was highest among jail inmates/STD clinic patients (10.5%) and family planning centers (10.4%), followed by family practice/internal medicine practices (7.8%) and ED (7.3%), and lowest in OB/GYN practices (4.4%). N. gonorrhoeae prevalence was highest in jail inmates/STD clinic patients (3.5%), followed by hospital-associated patients (2.2%), family planning centers (1.5%), and family practice/internal medicine practices (1.2%), and lowest in OB/GYN practices (0.78%).
Fig 3.
Prevalence rates by testing site. TV, Trichomonas vaginalis; CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae; Int Med, internal medicine; STD, sexually transmitted disease.
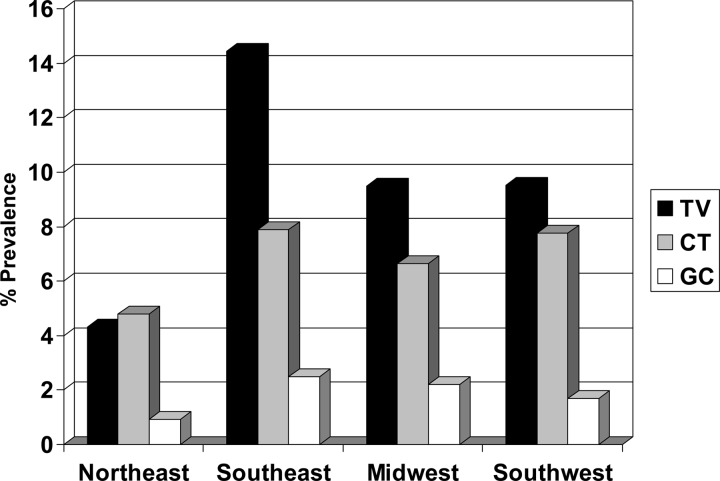
Fig 4.
Prevalence rates by geographical region. TV, Trichomonas vaginalis; CT, Chlamydia trachomatis; GC, Neisseria gonorrhoeae.
The subset of jail inmates had the overall highest rate of T. vaginalis (22.3%) and C. trachomatis (10.9%) infections, and the subset of inpatients had the highest overall rates of N. gonorrhoeae infections (4.0%). Univariate and multivariate analyses (Table 2) determined that T. vaginalis and N. gonorrhoeae infections were significantly associated with jail inmates/STD clinic attendees, while only T. vaginalis infections were significantly associated with women treated in hospital settings.
The highest U.S. rates for T. vaginalis, C. trachomatis, and N. gonorrhoeae were found in the Southeast (14.4%, 7.9%, and 2.5%, respectively), followed by the Southwest (9.5%, 7.8%, and 1.7%, respectively) and the Midwest (9.5%, 6.6%, and 2.2%, respectively). The Northeast had the lowest rates for all three STDs (T. vaginalis, 4.3%; C. trachomatis, 4.8%; N. gonorrhoeae, 0.9%). Univariate and multivariate analyses (Table 2) determined that the Southwest, Southeast, and Midwest were significantly associated with increased odds of T. vaginalis infections. The Southwest and Southeast were significantly associated with increased odds for C. trachomatis infections, and the Southeast and Midwest were significantly associated with increased odds for N. gonorrhoeae infections. By univariate analysis only, C. trachomatis infections were determined to be significantly associated with the Midwest and N. gonorrhoeae infections were determined to be significantly associated with the Southwest.
Prevalence of T. vaginalis, C. trachomatis, and N. gonorrhoeae infections by specimen type.
The prevalence of T. vaginalis, C. trachomatis, and N. gonorrhoeae infections by specimen type is presented in Table 3. Since only one sample type per patient was tested in this study, a direct comparison of detection rates by sample type was not possible. However, the overall prevalence rates for C. trachomatis, N. gonorrhoeae, T. vaginalis, and coinfections were equivalent for CVS and urine. The prevalence rates for C. trachomatis, N. gonorrhoeae, and T. vaginalis were slightly lower for endocervical swabs than for CVS and urine, but the rates for all samples were equivalent for coinfections. PCyt specimens had the lowest detection rates for C. trachomatis, N. gonorrhoeae, T. vaginalis, and coinfections.
Table 3.
Overall prevalence of T. vaginalis, C. trachomatis, N. gonorrhoeae, and mixed infections by specimen typea
| Specimen type | Prevalence (no. positive/no. tested [%]) of each infection |
||||||
|---|---|---|---|---|---|---|---|
| C. trachomatis | N. gonorrhoeae | T. vaginalis | C. trachomatis/N. gonorrhoeae/T. vaginalis | C. trachomatis/T. vaginalis | C. trachomatis/N. gonorrhoeae | N. gonorrhoeae/T. vaginalis | |
| Endocervical swab | 240/3,912 (6.13) | 57/3,911 (1.46) | 326/3,912 (8.33) | 11/3,911 (0.28) | 50/3,912 (1.28) | 23/3,911 (0.59) | 20/3,911 (0.51) |
| Liquid Pap transport | 23/940 (2.45) | 3/940 (0.32) | 32/940 (3.40) | 1/940 (0.11) | 3/940 (0.32) | 1/940 (0.11) | 1/940 (0.11) |
| Urine | 169/1,945 (8.69) | 51/1,942 (2.63) | 216/1,950 (11.08) | 4/1,940 (0.21) | 31/1,945 (1.59) | 16/1,940 (0.82) | 16/1,942 (0.82) |
| Vaginal swab | 76/791 (9.61) | 18/786 (2.29) | 89/791 (11.25) | 2/786 (0.25) | 13/791 (1.64) | 6/786 (0.76) | 9/786 (1.15) |
Specimen type results are not directly comparable, as only one specimen type per patient was tested.
DISCUSSION
Prevalence of T. vaginalis is not well characterized primarily because T. vaginalis infections are not reportable to public health officials and are generally not part of routine screening. In addition, published studies that measured T. vaginalis prevalence generally used relatively insensitive methods. This study is the first to use the highly sensitive (>95%) ATV to determine T. vaginalis prevalence in a large number of U.S. women of all ages undergoing screening or diagnostic testing for C. trachomatis/N. gonorrhoeae infections. The use of remnant samples allowed the comparison of T. vaginalis, C. trachomatis, and N. gonorrhoeae prevalences in various patient populations, clinical settings, and different geographical regions (21 states). Consecutive enrollment of all samples meeting inclusion criteria prevented sample selection bias.
Our study had several limitations that need to be considered when interpreting the data. The population is not nationally representative of the U.S. population in general, and no Pacific Northwest sites participated. Patient selection was biased (women were screened for C. trachomatis/N. gonorrhoeae), and the reasons for C. trachomatis/N. gonorrhoeae testing varied among age groups. Younger age groups (18 to <30 years old) were tested as part of routine screening (asymptomatic) or due to symptomatic complaints and therefore constituted a significant portion of the samples enrolled (4,882, versus 2,711 samples from women >30 years old). In contrast, women ≥50 years old were most likely symptomatic, as routine C. trachomatis/N. gonorrhoeae screening is not done in this age group. This study did not include sexually active women <18 years old. Studies by Goyal et al. determined the T. vaginalis prevalence to be 9.9% in women 14 to 19 years old who presented to the ED with complaints suggestive of an STI (12). Another study by Huppert et al. found that the T. vaginalis prevalence was 18.5% in women 14 to 21 years old (18). Due to the use of deidentified, discarded clinical samples, patient clinical status (asymptomatic or symptomatic), history of sexual partners, and history of STIs could not be obtained, thereby limiting the interpretation of the data. Therefore, the age of the patient contributes to the bias in STI prevalence noted in this study, and overall T. vaginalis rates may be underestimated by exclusion of certain populations. Finally, this study was not intended to compare the performances of the assays in the various specimen types, as multiple specimen types from each patient were not tested. However, overall, the detection rates for C. trachomatis, N. gonorrhoeae, and T. vaginalis were highly comparable in CVS and urine specimens and only slightly lower for ES. These specimen types accounted for 86.7% of the types tested, and comparable C. trachomatis, N. gonorrhoeae, and T. vaginalis detection rates suggest that no major disparities in assay performance by specimen type biased the overall results. As expected, the lowest detection rates for C. trachomatis, N. gonorrhoeae, and T. vaginalis were for PCyt specimens, as PCyt is used primarily for cervical cancer screening and STI testing is generally performed as adjunctive routine screening.
Despite these limitations, the following observations are of clinical significance. Strengths of the study included a substantial sample size, the availability of C. trachomatis/N. gonorrhoeae infection status, and the use of highly sensitive NAATs for T. vaginalis and C. trachomatis/N. gonorrhoeae testing from the same sample.
T. vaginalis prevalence was 8.7% in the overall study population. This value is higher than the 3.2% value reported in the 2001 to 2004 NHANES, the most recent study reporting T. vaginalis prevalence in the general U.S. female population (1). The difference in prevalence between this study and NHANES may be attributed to various factors. First, it may be due to an increase in T. vaginalis prevalence in the last 6 to 9 years (NHANES utilized data from 2001 to 2004). Second, the higher sensitivity of ATV compared to that of the PCR used in NHANES allows the detection of a higher number of cases and therefore may have yielded a higher prevalence. Third, and most important, the populations analyzed in NHANES and the present study differed. The NHANES population consisted of noninstitutionalized civilians selected at random that represent the entire U.S. population because the data set was weighted based on U.S. Census data and was generally a low-risk population. In contrast, our study population was inherently at higher risk for STIs since the subjects were undergoing testing for C. trachomatis/N. gonorrhoeae. It is unknown what percentage of the patients were asymptomatic for C. trachomatis/N. gonorrhoeae and being screened according to current guidelines (5, 35) or were symptomatic for C. trachomatis/N. gonorrhoeae and being tested for diagnosis of C. trachomatis/N. gonorrhoeae. Regardless of the symptomatic status, this population can be considered potentially high risk for STIs since the CDC and other guidelines recommend annual C. trachomatis/N. gonorrhoeae screening of sexually active women under 26 years or older women with risk factors, such as new sexual partners. In addition, our population was 18.2% black (which is larger than the national average of 12.8%) (30), a population known to have high T. vaginalis prevalence (13% to 14%) (1, 29).
Like NHANES, we found that T. vaginalis prevalence was higher than that for C. trachomatis and N. gonorrhoeae combined, even though C. trachomatis and N. gonorrhoeae prevalence in our population was higher than that reported in NHANES (2.2% for C. trachomatis and 0.24% for N. gonorrhoeae) (9). A recent prevalence study, in which home-collected mailed vaginal swabs were tested by ATV, reported a 10% prevalence of T. vaginalis in women (11). Unlike our study, that report did not find that age was significantly associated with T. vaginalis positivity. Like the present study, however, black race was highly associated with T. vaginalis infection (adjusted OR, 2.69; prevalence, 13.2%).
An analysis of T. vaginalis prevalence by age group revealed that T. vaginalis infection affects women of all ages and that T. vaginalis prevalence is highest in women >40 years old and lowest in women <40 years old. These findings are in agreement with NHANES (1, 29) and other studies (3, 10, 23, 27). This age distribution contrasts with that of C. trachomatis and N. gonorrhoeae, whose prevalence was highest in women <30 years old and lowest in women ≥40 years old (9). T. vaginalis prevalence remained higher than that of C. trachomatis and N. gonorrhoeae for all age groups except the youngest women (18 to 19 years), for whom C. trachomatis prevalence was highest. This trend agrees with a previous study that reported C. trachomatis, T. vaginalis, and N. gonorrhoeae prevalence rates of 10.1%, 6.0%, and 4.1%, respectively, in women 14 to 17 years old (32) and with results from another study that showed prevalence rates of 11%, 6.3%, and 0%, respectively, in women 13 to 21 years old (16). Rates of T. vaginalis, C. trachomatis, and N. gonorrhoeae coinfection were low (<1.3%) in the whole population. Other studies reported coinfection rates in high-risk populations or reported C. trachomatis and N. gonorrhoeae prevalence in T. vaginalis-positive women (1, 24). T. vaginalis/C. trachomatis and T. vaginalis/N. gonorrhoeae coinfections were more common in women <30 years old, probably because C. trachomatis and N. gonorrhoeae infections are more prevalent in this age group.
An analysis of STI prevalence by ethnicity showed large disparities. T. vaginalis, C. trachomatis, and N. gonorrhoeae prevalences were highest in black populations, a trend documented in the U.S. population for T. vaginalis as well as for C. trachomatis and N. gonorrhoeae (1, 9, 11, 15, 17, 29). NHANES found the highest T. vaginalis rates in non-Hispanic black women (13.3%), and within this group, T. vaginalis rates increased from 8.3% (14 to 19 years) to almost 20% (40 to 49 years) (1). A significant association between black race and T. vaginalis prevalence was reported for high-risk adolescent females (16). Although other ethnic groups (American Indians/Alaskan Natives and Native Hawaiians/Pacific Islanders) had high T. vaginalis prevalence rates, due to low subject numbers, we cannot conclude that this prevalence is truly representative in these ethnic groups.
We found that T. vaginalis prevalence and, in some cases, C. trachomatis and N. gonorrhoeae prevalence were highest in women attending STD clinics, testing in a hospital setting, and incarcerated in jails. High T. vaginalis prevalence (32%) has been observed among women incarcerated in San Francisco county jail (10). Similar findings relating to C. trachomatis and N. gonorrhoeae infections were reported for black adolescents 18 to 19 years old who were incarcerated in adult correction facilities (19). Although women attending STD clinics are expected to have high T. vaginalis, C. trachomatis, and N. gonorrhoeae rates due to high-risk behavior and/or symptomatic status, providers should be aware of the high T. vaginalis rates in the hospital setting. Patients who are presenting with symptomatic vaginal complaints and using the ED as the primary source of health care may contribute to this high rate and will require further investigation. Interestingly, corresponding C. trachomatis and N. gonorrhoeae rates were not particularly high in this setting. This lower prevalence in OB/GYN, family practice/internal medicine, and family planning clinics might be expected since many of these women were probably being routinely screened according to C. trachomatis/N. gonorrhoeae testing guidelines and were <26 years old and/or pregnant. Interestingly, women in family planning and family practice groups had higher rates of C. trachomatis than T. vaginalis.
In summary, the availability of the FDA-cleared ATV, which can be run on the same fully automated system and with the same samples as AC2, provides a unique opportunity to screen women at risk for Trichomonas vaginalis, Chlamydia trachomatis, and Neisseria gonorrhoeae infections. High T. vaginalis prevalence in all age groups suggests that all women that are considered at high risk for STIs (multiple or new partners, non-barrier contraception, etc.), including women >40 years old, should be screened for T. vaginalis even if they are not being tested for C. trachomatis/N. gonorrhoeae. T. vaginalis screening can be performed when these women appear for routine adjunct human papillomavirus (HPV)/cytology screening since all testing can be done using the same PCyt sample or a separate swab sample. The high prevalence of T. vaginalis infection, particularly in asymptomatic women, emphasizes the need for T. vaginalis to be made a reportable disease by the CDC. Further studies using ATV are needed to estimate the true prevalence of T. vaginalis in adolescent females younger than 18 years of age.
ACKNOWLEDGMENTS
We sincerely thank the following site principal investigators and technical staff for their support of this study: S. Beqaj, DCL, Indianapolis, IN; T. Drake, Grady Memorial Hospital, Atlanta, GA; M. Ervin, DOH, Columbus, OH; A. Valsamakis and N. Quinn, Johns Hopkins University School of Medicine, Baltimore, MD; J. Getchell, Delaware Health & Social Services DPH Laboratory, Smyrna, DE; G. Hansen, Hennepin County Medical Center, Minneapolis, MN; P. Kerndt and A. Stirland, LA County STD Program, Los Angeles, CA; K. Kronquist, Kaiser Permanente Regional Reference Laboratory, Denver, CO; J. Lovchik, Indiana State Department of Health, Indianapolis, IN; J. Matthews-Greer, LSU Health Science Center, Shreveport, LA; K. Murphy, Propath, Dallas, TX; S. Novak-Weekley, Kaiser North Hollywood, North Hollywood, CA; M. Pandori, San Francisco DOH, San Francisco, CA; A. Rao, Scott and White Hospital, Temple, TX; L. Samuel, Henry Ford Hospital, Detroit, MI; A. Wagner, Women's OB/Saginaw Valley Medical Research, Saginaw, MI; and S. Young, TriCore Reference Laboratories, Albuquerque, NM. We thank Florence Paillard for her editorial assistance.
This study was funded by Gen-Probe, Inc. All reagents and supplies were provided by Gen-Probe. This study was funded in part by NIH HPTN U-01 AI068613 and U-54EB007958.
C.C.G. and K.C. are members of the Gen-Probe Scientific Advisory Board and have received research funding from Gen-Probe. C.A.G. and J.S.S. have received research funding from Gen-Probe. C.S.H. and J.S. are employees of Gen-Probe.
Footnotes
Published ahead of print 23 May 2012
REFERENCES
- 1. Allsworth JE, Ratner JA, Peipert JF. 2009. Trichomoniasis and other sexually transmitted infections: results from the 2001-2004 National Health and Nutrition Examination Surveys. Sex. Transm. Dis. 36:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American College of Obstetricians and Gynecologists (ACOG) 2006. ACOG practice bulletin. Clinical management guidelines for obstetrician-gynecologists, no. 72: vaginitis. Obstet. Gynecol. 107:1195–1206 [DOI] [PubMed] [Google Scholar]
- 3. Andrea SB, Chapin KC. 2011. Comparison of Aptima Trichomonas vaginalis transcription-mediated amplification and BD Affirm VPIII for detection of T. vaginalis in symptomatic women: performance parameters and epidemiologic implications. J. Clin. Microbiol. 49:866–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Briselden AM, Hillier SL. 1994. Evaluation of Affirm VP Microbial Identification Test for Gardnerella vaginalis and Trichomonas vaginalis. J. Clin. Microbiol. 32:148–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention (CDC) 2011. CDC Grand Rounds: chlamydia prevention: challenges and strategies for reducing disease burden and sequelae. MMWR Morb. Mortal. Wkly. Rep. 60:370–373 [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) CDC fact sheet: trichomoniasis. 2010. http://www.cdc.gov/std/trichomonas/STDFact-Trichomoniasis.htm.
- 7. Cherpes TL, et al. 2006. The associations between pelvic inflammatory disease, Trichomonas vaginalis infection, and positive herpes simplex virus type 2 serology. Sex. Transm. Dis. 33:747–752 [DOI] [PubMed] [Google Scholar]
- 8. Cotch MF, et al. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. Sex. Transm. Dis. 24:353–360 [DOI] [PubMed] [Google Scholar]
- 9. Datta SD, et al. 2007. Gonorrhea and chlamydia in the United States among persons 14 to 39 years of age, 1999 to 2002. Ann. Intern. Med. 147:89–96 [DOI] [PubMed] [Google Scholar]
- 10. Freeman AH, et al. 2010. Prevalence and correlates of Trichomonas vaginalis among incarcerated persons assessed using a highly sensitive molecular assay. Sex. Transm. Dis. 37:165–168 [DOI] [PubMed] [Google Scholar]
- 11. Gaydos CA, et al. 2011. Trichomonas vaginalis infection in women who submit self-obtained vaginal samples after internet recruitment. Sex. Transm. Dis. 38:828–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goyal M, Hayes K, McGowan KL, Fein JA, Mollen C. 2011. Prevalence of Trichomonas vaginalis infection in symptomatic adolescent females presenting to a pediatric emergency department. Soc. Acad. Emerg. Med. 18:763–766 [DOI] [PubMed] [Google Scholar]
- 13. Hardick A, Hardick J, Wood BJ, Gaydos C. 2006. Comparison between the Gen-Probe transcription-mediated amplification Trichomonas vaginalis research assay and real-time PCR for Trichomonas vaginalis detection using a Roche LightCycler instrument with female self-obtained vaginal swab samples and male urine samples. J. Clin. Microbiol. 44:4197–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hardick J, Yang S, Lin S, Duncan D, Gaydos C. 2003. Use of the Roche LightCycler instrument in a real-time PCR for Trichomonas vaginalis in urine samples from females and males. J. Clin. Microbiol. 41:5619–5622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Helms DJ, et al. 2008. Risk factors for prevalent and incident Trichomonas vaginalis among women attending three sexually transmitted disease clinics. Sex. Transm. Dis. 35:484–488 [DOI] [PubMed] [Google Scholar]
- 16. Hollman D, Coupey SM, Fox AS, Herold BC. 2010. Screening for Trichomonas vaginalis in high-risk adolescent females with a new transcription-mediated nucleic acid amplification test (NAAT): associations with ethnicity, symptoms, and prior and current STIs. J. Pediatr. Adolesc. Gynecol. 23:312–316 [DOI] [PubMed] [Google Scholar]
- 17. Hughes G, et al. 2000. Comparison of risk factors for four sexually transmitted infections: results from a study of attendees at three genitourinary medicine clinics in England. Sex. Transm. Infect. 76:262–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huppert JS, et al. 2007. Rapid antigen testing compares favorably with transcription-mediated amplification assay for the detection of Trichomonas vaginalis in young women. Clin. Infect. Dis. 45:194–198 [DOI] [PubMed] [Google Scholar]
- 19. Joesoef MR, Kahn RH, Weinstock HS. 2006. Sexually transmitted diseases in incarcerated adolescents. Curr. Opin. Infect. Dis. 19:44–48 [DOI] [PubMed] [Google Scholar]
- 20. Kissinger P, et al. 2009. Trichomonas vaginalis treatment reduces vaginal HIV-1 shedding. Sex. Transm. Dis. 36:11–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madico G, Quinn TC, Rompalo A, McKee KT, Jr, Gaydos CA. 1998. Diagnosis of Trichomonas vaginalis infection by PCR using vaginal swab samples. J. Clin. Microbiol. 36:3205–3210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McClelland RS, et al. 2007. Infection with Trichomonas vaginalis increases the risk of HIV-1 acquisition. J. Infect. Dis. 195:698–702 [DOI] [PubMed] [Google Scholar]
- 23. Miller WC, et al. 2005. The prevalence of trichomoniasis in young adults in the United States. Sex. Transm. Dis. 32:593–598 [DOI] [PubMed] [Google Scholar]
- 24. Munson E, et al. 2008. Impact of Trichomonas vaginalis transcription-mediated amplification-based analyte-specific-reagent testing in a metropolitan setting of high sexually transmitted disease prevalence. J. Clin. Microbiol. 46:3368–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nye MB, Schwebke JR, Body BA. 2009. Comparison of APTIMA Trichomonas vaginalis transcription-mediated amplification to wet mount microscopy, culture, and polymerase chain reaction for diagnosis of trichomoniasis in men and women. Am. J. Obstet. Gynecol. 200:188.e1-188.e7 doi:10.1016/j.ajog.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 26. Radonjic IV, et al. 2006. Diagnosis of Trichomonas vaginalis infection: the sensitivities and specificities of microscopy, culture and PCR assay. Eur. J. Obstet. Gynecol. Reprod. Biol. 126:116–120 [DOI] [PubMed] [Google Scholar]
- 27. Schwebke JR, Burgess D. 2004. Trichomoniasis. Clin. Microbiol. Rev. 17:794–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schwebke JR, et al. 2011. Molecular testing for Trichomonas vaginalis in women; results from a prospective U.S. clinical trial. J. Clin. Microbiol. 49:4106–4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sutton M, et al. 2007. The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001-2004. Clin. Infect. Dis. 45:1319–1326 [DOI] [PubMed] [Google Scholar]
- 30.United States Census 2010. 2010. [Accessed 5 April 2011]. http://2010.census.gov/2010census/data/
- 31. Van Der Pol B, et al. 2008. Trichomonas vaginalis infection and human immunodeficiency virus acquisition in African women. J. Infect. Dis. 197:548–554 [DOI] [PubMed] [Google Scholar]
- 32. Van Der Pol B, Williams JA, Orr DP, Batteiger BE, Fortenberry JD. 2005. Prevalence, incidence, natural history, and response to treatment of Trichomonas vaginalis infection among adolescent women. J. Infect. Dis. 192:2039–2044 [DOI] [PubMed] [Google Scholar]
- 33. Van der Pol B. 2007. Trichomonas vaginalis infection: the most prevalent nonviral sexually transmitted infection receives the least public health attention. Clin. Infect. Dis. 44:23–25 [DOI] [PubMed] [Google Scholar]
- 34. Wendel KA, Erbelding EJ, Gaydos CA, Rompalo AM. 2002. Trichomonas vaginalis polymerase chain reaction compared with standard diagnostic and therapeutic protocols for detection and treatment of vaginal trichomoniasis. Clin. Infect. Dis. 35:576–580 [DOI] [PubMed] [Google Scholar]
- 35. Workowski KA, Berman SM, Centers for Disease Control and Prevention 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recommend. Rep. 59(RR-12):1–110 [PubMed] [Google Scholar]