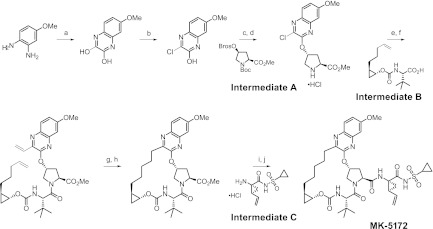
Fig 2.
Synthetic route and chemical structure of MK-5172. The reagents used are as follows: (a) diethyloxalate, triethylamine, 150°C; (b) thionyl chloride, dimethylformamide, 110°C; (c) cesium carbonate, 1-methyl-2-pyrrolidinone, intermediate A (Boc, t-butoxycarbonyl; Bros, 4-bromophenyl sulfonyl); (d) HCl, dioxane; (e) O-(7-azabenzotriazol-7-yl)-N,N,N′,N'-tetramethyluronium hexafluorophosphate, diisopropylethylamine, dimethylformamide, intermediate B; (f) potassium vinyltrifluoroborate, triethylamine, dichloro[1,1-bis(diphenylphosphino)ferrocene]palladium (II) chloride, ethanol; (g) Zhan 1B catalyst, 1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidene[2-(i-propoxy)-5-(N,N-dimethylaminosulfonyl)phenyl] methyleneruthenium (II) dichloride (CAS 918870-76-5), 1,2-dichloroethane; (h) hydrogen, 10% Pd/C, methanol, dioxane; (i) lithium hydroxide, tetrahydrofuran, tetrahydrofuran, water; (j) O-(benzotriazol-1-yl)-N,N,N′,N'-tetramethyluronium tetrafluoroborate, diisopropylethylamine, 4-dimethylamino pyridine, dichloromethane, intermediate C.