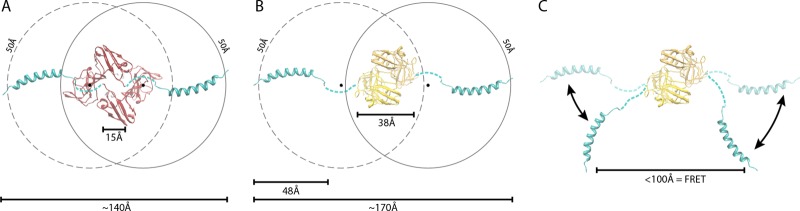
Fig 6.
Model of NS5A domain 1 dimer in a FRET-competent state. The solution structure of the N-terminal amphipathic helix PDB 1R7E (26) was modeled onto the crystal structures of two NS5A domain 1 dimer configurations. Structures are oriented to view the proposed membrane-binding surface of NS5A. Models and distance measurement were created with PDB 1ZH1 (33) (A) and PDB 3FQM (B) (23). The models were constructed with the N termini separated by a maximal distance assuming a flexible linker between the N-terminal helix and domain 1, consistent with the disorder in this region of the crystal structures. Shown are the distances between the N termini of the crystal structure monomers for the amphipathic helix, the maximal length between the modeled termini, the 48-Å length of the amphipathic helix, and a hypothetical 50-Å radius assuming rotation of the helix around the flexible linker. The midpoint of this linker is indicated by a black spot. In this configuration, the distance between N termini exceeds the range compatible with FRET. (C) Potential rotation of the N-terminal helix around the flexible linker in the plane of the membrane readily positions the termini in a FRET-competent state without causing clashes with the core of domain 1 for PDB 3FQM and 1ZH1 (not shown).