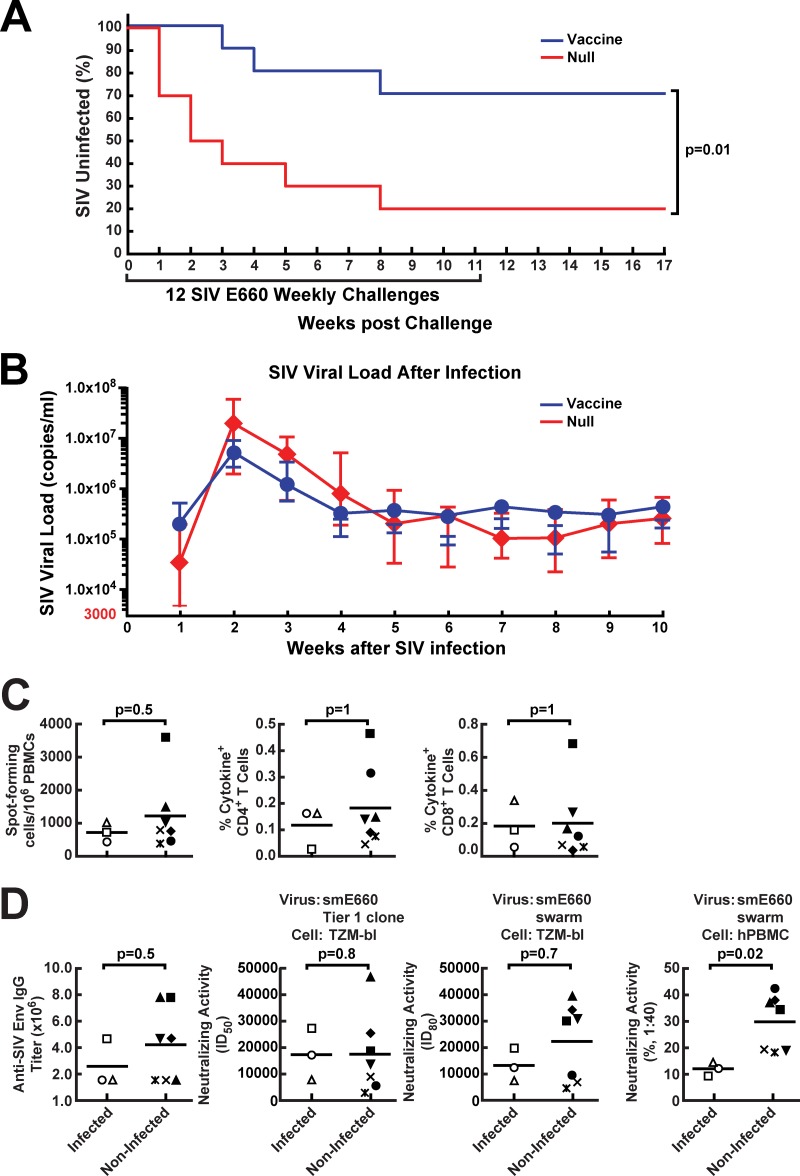
Fig 7.
Protection of immunized monkeys against acquisition of SIV smE660 infection and correlates of protection in vaccinated animals. (A) Kaplan-Meier curves for SIV acquisition are shown for the 10 monkeys which were vaccinated using rAd5 encoding SIV Env and then boosted 8 weeks later with an LCMV vector encoding SIV Env in comparison with mock (null vector)-vaccinated animals. Weekly challenges were discontinued in infected animals upon detection of SIV loads in plasma. The reduction in protection per challenge was 82%, while cumulative protection over the course of the study was 62% (P = 0.01 by the log rank test). (B) For animals that acquired SIV infection during the challenge period independent of whether vaccinated or not, peak plasma SIV viral loads were recorded each week upon detection. Geometric means with standard errors of the means (SEMs) are plotted for 8 control animals and 3 vaccinated animals that were infected. (C and D) At week 11, whole blood in EDTA or serum samples were obtained from vaccinated animals. SIV-specific cellular immune responses in PBMC were quantified after in vitro stimulation with Env peptide pools and were analyzed by either ELISpot formation or cytokine production of CD4+ and CD8+ T cells. (C) Cellular immune responses of infected and noninfected vaccinated monkeys were analyzed. (D) The serum samples were tested for the presence of SIV Env binding antibodies (ELISA), or neutralizing activity against an SIV smE660 tier 1 clone or smE660 swarm was assessed using TZM-bl cells or human PBMC. The two groups were then compared using statistical analysis. In panels C and D, each symbol represents one individual animal.