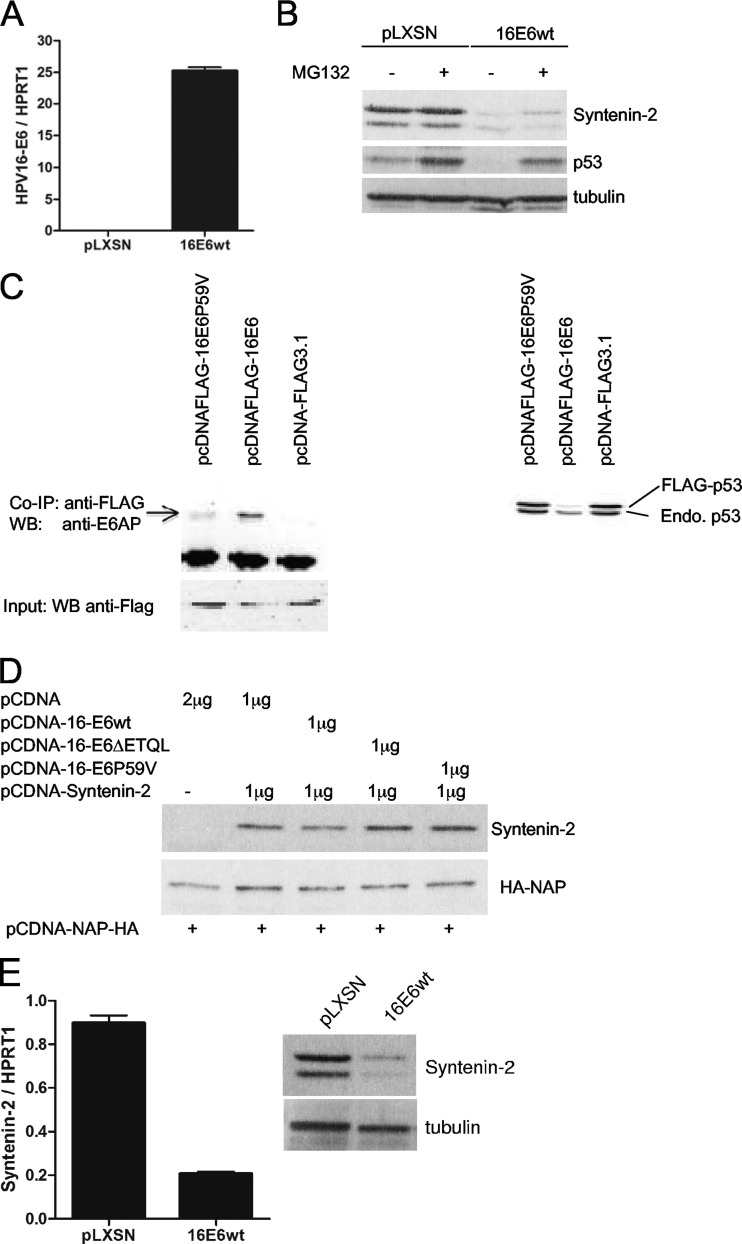
Fig 3.
Syntenin-2 is targeted by HPV16 E6 at the transcriptional level. (A) PHEK expressing HPV16 E6 wt were studied for E6 mRNA expression by qRT-PCR. (B) HPV16 E6-positive PHEK were incubated in the presence of either MG132 or solvent before harvesting and then analyzed by Western blotting. (C) Coimmunoprecipitation (Co-IP) showed that the HPV16 E6P59V mutant does not bind to E6AP (left). Extracts from C33a cells, transiently transfected into 10-cm culture dishes with empty vectors or vectors for HPV16 E6-Flag or HPV16 E6P59V-Flag (15 μg each) were incubated with anti-Flag–M2 affinity beads. Bound E6AP was detected in the subsequent Western blot analysis by using a monoclonal E6AP antibody. To verify the presence of Flag-tagged protein, an aliquot of the extract was used as input control in a Western blot developed with the M2 anti-Flag antibody. In vivo degradation assay showed that the HPV16 E6P59V mutant does not degrade p53 (right). C33a cells were transiently cotransfected into 6-cm culture dishes with expression vectors for Flag-p53 and vectors coding for HPV16 E6-Flag or HPV16 E6P59V-Flag (2.5 μg each), and then p53 levels were detected by Western blotting using the Do-1 anti-p53 antibody. (D) Transient transfection of C33a with HPV16 E6 expression vectors was performed as indicated. Additionally, transfected HA-NAP was included as an internal transfection control. (E) Syntenin-2 mRNA expression was quantified in pLXSN- and HPV16 E6-positive PHEK by qRT-PCR, and protein levels detected by Western blotting. MRNA levels of gene targets were normalized to HPRT1 mRNA levels. Error bars show standard deviations.