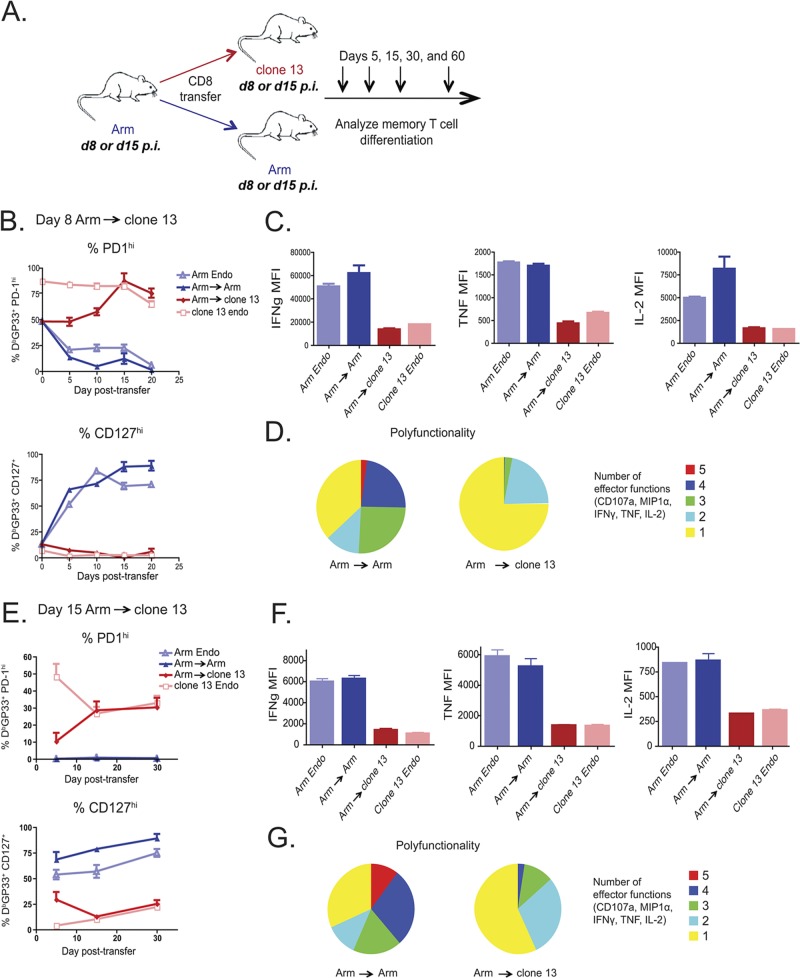
Fig 4.
Optimal priming does not prevent exhaustion of effector CD8 T cells transferred to a chronic infection. (A) Experimental design. Ly5.1+ CD8 T cells were purified from either day 8 or day 15 Arm-infected mice and adoptively transferred (day 8, ∼5 × 105 DbGP33+ CD8 T cells/recipient; day 15, ∼1 × 105 DbGP33+ CD8 T cells/recipient) to infection-matched clone 13- or Arm-infected recipient mice. Donor virus-specific CD8 T cells were examined on days 5, 15, 30, and 60 p.t. in recipient spleens. (B to D) Day 8 Arm → clone 13. (B) Percentage of Ly5.1+ DbGP33+ CD8 T cells expressing CD127 or high levels of PD-1 in the blood; (C) mean fluorescence of IFN-γ, IL-2, and TNF expressed by CD8 T cells restimulated ex vivo with the GP33 peptide on day 60 p.t.; (D) polyfunctionality of day 8 Arm → Arm and day 8 Arm → clone 13. Colors represent the number of cytokines (1 to 5) coproduced per cell. (E to G) Day 15 Arm → clone 13. (E) Percentage of Ly5.1+ DbGP33+ CD8 T cells expressing CD127 or high levels of PD-1 in the blood; (F) mean fluorescence of IFN-γ, IL-2, and TNF expressed by CD8 T cells restimulated ex vivo with the GP33 peptide on day 60 p.t.; (G) polyfunctionality of day 15 Arm → Arm and day 15 Arm → clone 13 displayed as in panel D. MFI, mean fluorescence intensity; MIP1α, macrophage inflammatory protein-1α.