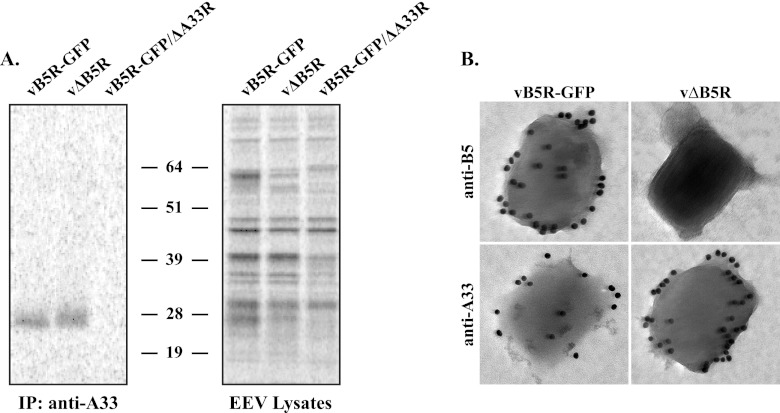
Fig 2.
Incorporation of A33 into EEV does not require B5. (A) Immunoprecipitation (IP). Lysates of purified radiolabeled EEV released from cells infected with the indicated viruses were immunoprecipitated with an anti-A33 MAb. Protein-antibody complexes were resolved by SDS-PAGE, and proteins were detected by autoradiography. Equilibrated EEV lysates were analyzed to verify that equal amounts of EEV were used for immunoprecipitation. The molecular masses, in kilodaltons, and positions of marker proteins are shown. (B) Immunoelectron microscopy. EEV released from cells infected with the indicated viruses were stained with either an anti-B5 MAb or an anti-A33 MAb, followed by 18-nm colloidal gold-conjugated goat anti-rat or anti-mouse antibody, respectively. Immunogold-labeled EEV were negatively stained and visualized using a Hitachi 7650 TEM.