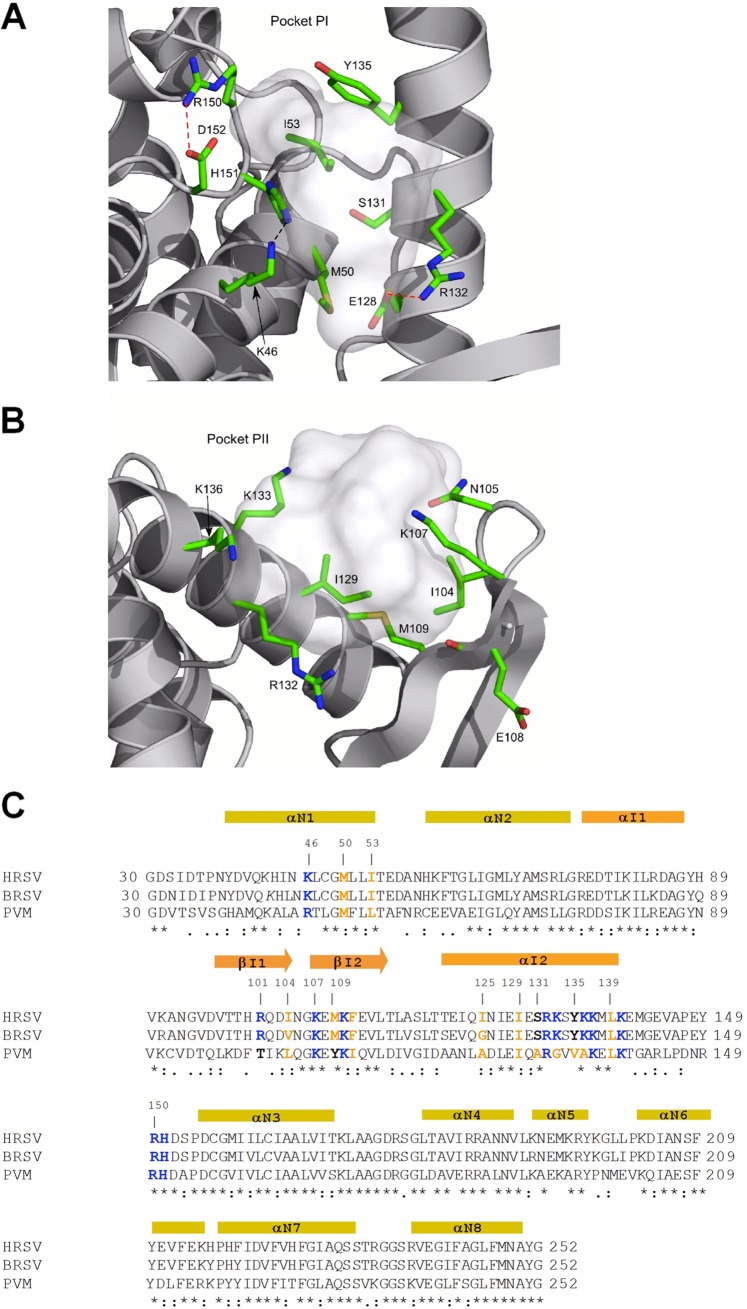
Fig 4.
Close-up of PI and PII. (A) PI is defined by hydrophobic side chains and hydrogen bond acceptors and donors positioned on rigid fragments of N. Hydrogen bonds and salt bridges are represented by black and red dotted lines, respectively. (B) PII is hosted between an α-helix and the flexible turn of a β-sheet. (C) Sequence alignment of NNTDs of HRSV, BRSV, and PVM by ClustalW2. Strictly conserved residues are indicated by stars, and partially conserved residues are indicated by points under the alignment. Secondary-structure elements are indicated above the sequence, shown in yellow according to the NNTD and in orange for the variable region (44). Residues mutated in this study and potentially implicated in PCTD-N interactions are indicated in boldface type and colored according to their biochemical properties (blue, basic residues; orange, hydrophobic residues; black, neutral residues).