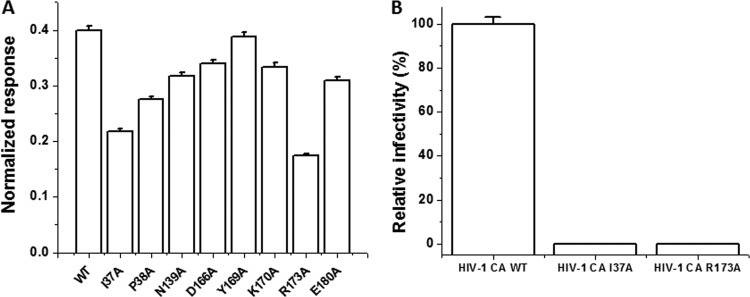
Fig 6.
(A) Effect of mutation of capsid residues in and around the proposed I-XW-053 binding site on compound binding. The interaction of I-XW-053 at a concentration of 27.5 μM with wild-type and mutant versions of the CA protein was assessed using SPR. To allow comparison, responses at equilibrium were normalized to the theoretical Rmax, assuming a 2:1 interaction. (B) Infection efficiency of mutations made within the HIV-1NL4-3 CA provirus. 293T cells were transfected with either wild-type pNL4-3 CA or pNL4-3 backbone containing a single amino acid change within CA (Ile37Ala or Arg173Ala). Pseudoviral supernatants were normalized to reverse transcriptase content before infection of U87.CD4.CXCR4 target cells. Results were measured by luciferase activity and were normalized to the infectivity of wild-type pNL4-3 CA and plotted, with standard deviations shown by error bars. Note that the standard deviation for the I37A mutant was 0.002 and that for the R173A mutant was 0.001 (not visible on the histograms).