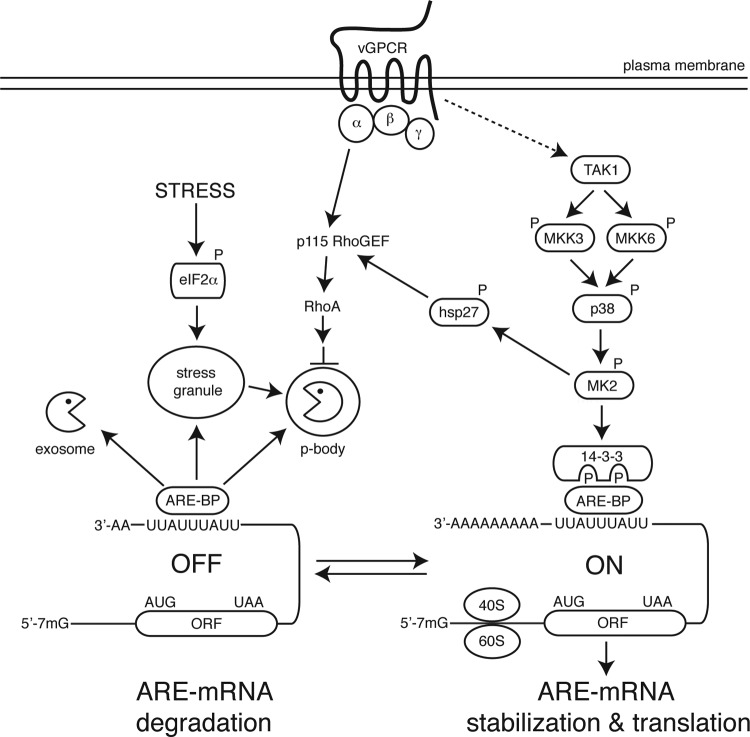
Fig 7.
Model of the mechanism of vGPCR-mediated ARE-mRNA stabilization. In this working model, vGPCR stimulates two signal transduction pathways that negatively impact ARE-mRNA decay. vGPCR has been reported to promote the phosphorylation and polyubiquitination of TAK1 (9), which initiates a signal transduction pathway involving activation of MKK3 and/or MKK6 (47), p38 MAPK, and the p38 substrate MK2. MK2 phosphorylates destabilizing ARE-binding proteins like tristetraprolin (TTP), generating binding sites for cytoplasmic 14-3-3 scaffolding proteins that disrupt recruitment of a complex of endo- and exonucleases known as the exosome (15, 39). In addition, MK2 phosphorylates the heat shock protein 27 (Hsp27), which has been shown to form a complex with p115GEF to activate RhoA (27). In response to a variety of stresses, including viral infection, eukaryotic translation initiation factor 2α (eIF2α) is phosphorylated, which prevents initiation by limiting the availability of eIF2-GTP-tRNAmet. Specific RNA binding proteins bind to these stalled translation preinitiation complexes and nucleate the formation of large cytoplasmic mRNP aggregates known as stress granules (SGs), in which translationally inactive transcripts are triaged and routed to sites of reinitiation or degradation (2). TTP has been reported to transport bound ARE-mRNAs to processing bodies (PBs), sites of translational repression, and mRNA degradation (35). vGPCR activates RhoA (43, 57), and recent work has shown that RhoA activation disrupts the stress-induced rearrangement of PBs and stabilizes ARE-mRNAs (62). We provide evidence that vGPCR impacts both MK2 activity and PB dynamics, thereby potently stabilizing ARE-mRNAs and increasing the production of proteins encoded by ARE-mRNAs (e.g., Cox-2, IL-6). These studies provide the first evidence for vGPCR-mediated reprogramming of host gene expression at the posttranscriptional level, which may facilitate secretion of angiogenic growth factors from lytically infected cells.