Fig 5.
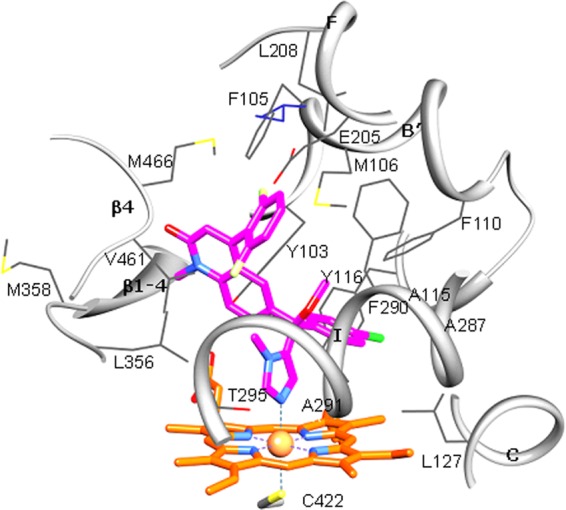
Active site of Trypanosoma brucei CYP51 with bound compound 3. The residues located within van der Waals contacts (<4.5 Å) with the inhibitor are shown in wire representation and labeled, carbon atoms are colored in gray, and the corresponding secondary structural elements of the protein are presented as ribbons. The single active-site amino acid residue that differs in T. cruzi CYP51 (I105) is colored in purple. Coordinated with the heme (orange), compound 3 is colored in magenta. Nitrogens are blue, oxygens are red, chlorine is green, and fluorines are light yellow. A stereoview of the active site in the superimposed T. brucei and T. cruzi (3K1O) CYP51 structures can be seen as Fig. S2 in the supplemental material.
