Abstract
Heterotrimeric protein phosphatase 2A (PP2A) consists of catalytic C (PP2Ac), structural A, and regulatory B-type subunits, and its dysfunction has been linked to cancer. Reversible methylation of PP2Ac by leucine carboxyl methyltransferase 1 (LCMT-1) and protein phosphatase methylesterase 1 (PME-1) differentially regulates B-type subunit binding and thus PP2A function. Polyomavirus middle (PyMT) and small (PyST) tumor antigens and SV40 small tumor antigen (SVST) are oncoproteins that block PP2A function by replacing certain B-type subunits, resulting in cellular transformation. Whereas the B-type subunits replaced by these oncoproteins seem to exhibit a binding preference for methylated PP2Ac, PyMT does not. We hypothesize that circumventing the normal cellular control of PP2A by PP2Ac methylation is a general strategy for ST- and MT-mediated transformation. Two predictions of this hypothesis are (1) that PyST and SVST also bind PP2A in a methylation-insensitive manner and (2) that down-regulation of PP2Ac methylation will activate progrowth and prosurvival signaling and promote transformation. We found that SVST and PyST, like PyMT, indeed form PP2A heterotrimers independently of PP2Ac methylation. In addition, reducing PP2Ac methylation through LCMT-1 knockdown or PME-1 overexpression enhanced transformation by activating the Akt and p70/p85 S6 kinase (S6K) pathways, pathways also activated by MT and ST oncoproteins. These results support the hypothesis that MT and ST oncoproteins circumvent cellular control of PP2A by methylation to promote transformation. They also implicate LCMT-1 as a negative regulator of Akt and p70/p85 S6K. Therefore, disruption of PP2Ac methylation may contribute to cancer, and modulation of this methylation may serve as an anticancer target.
Introduction
Protein phosphatase 2A (PP2A) is a major serine/threonine phosphatase found in all eukaryotic cells, which regulates numerous cellular events related to normal growth, including but not limited to cell proliferation, survival, and translation [1]. PP2A is typically a negative regulator of these processes, preventing uncontrolled cell growth and, consequently, cancer. PP2A most commonly exists as a heterotrimer composed of a structural A subunit, a catalytic C subunit (PP2Ac), and a regulatory B-type subunit. With two distinct isoforms of both the A and C subunits (α and β) and more than 20 regulatory B-type subunits encompassing four largely unrelated families (B/PPP2R2, B′/PPP2R5, B″/PPP2R3, and B‴/STRN), more than 80 different PP2A holoenzymes may exist [2]. This large number of heterotrimeric forms facilitates PP2A-mediated regulation of a wide variety of substrates and pathways, including the growth and survival-related Akt, p70/p85 S6 kinase (S6K), and mitogen-activated protein kinase/extracellular signal-regulated kinase pathways, among many others [3,4].
PP2A heterotrimer formation is regulated in part by the reversible methylation of the α-carboxyl group of the PP2A catalytic C subunit carboxyl-terminal leucine 309 (L309) [5–8]. Leucine carboxyl methyltransferase 1 (LCMT-1) methylates PP2Ac [9], whereas protein phosphatase methylesterase 1 (PME-1) serves as the PP2Ac demethylase [10,11]. The methylation status of PP2Ac alters the composition of PP2A by differentially modulating the recruitment of certain regulatory B-type subunits to the core A/C heterodimer [5–8]. For example, PP2Ac methylation is required for the efficient formation of heterotrimers containing the Bα subunit [5,8] and enhances heterotrimer formation for some B′ family members [5,7,12]; however, PP2Ac methylation is not necessary for the formation of heterotrimers containing the B‴ B-type subunits [8]. Therefore, control of PP2Ac methylation through LCMT-1 and PME-1 is important for regulating the formation and function of PP2A heterotrimers containing methylation-sensitive B-type subunits, that is, B-type subunits whose incorporation into PP2A heterotrimers is enhanced by methylation of PP2Ac. Given that these methylation-sensitive B-type subunits have been implicated in control of cell growth and survival (for examples, see References [13–16]), one important role of PP2Ac methylation may be to coordinately regulate multiple B-type subunits to negatively regulate cell proliferation and survival. If this is the case, LCMT-1 may be important for PP2A's tumor suppressive function. However, the relevance of LCMT-1 to oncogenic transformation has not been explored.
PP2A was first specifically implicated in cancer when it was identified as an important target of polyomavirus middle (PyMT) and small (PyST) tumor antigens and SV40 small tumor antigen (SVST) [17]. The middle (MT) and small (ST) tumor antigens function as viral B-type subunits, substituting for, and thus inhibiting the function of, certain cellular PP2A B-type subunits [17–21]. MT and ST oncoproteins only replace a portion of B-type subunits in a transformed cell [18,22,23]. Therefore, it is important to identify the nature of the cellular B-type subunits that are replaced by MT and ST oncoproteins. While these efforts are still at an early stage, three B-type subunits, Bα, B′α, and B′γ, have been identified as being targeted for replacement by MT and/or ST oncoproteins [17,21,23,24]. For example, SVST replaces Bα [21] and B′α and B′γ [23], consequently activating the extracellular signal-regulated kinase [21], Akt [25–27], and nuclear factor κB [28,29] signaling pathways to promote unrestricted growth and survival and thus transformation.
Interestingly, of the three cellular B-type subunits reported to be displaced by the MT or ST oncoproteins, two (Bα and B′α) are methylation-sensitive and one (B′γ) is likely to be methylation-sensitive [5,7,8,12,30]. An important question is whether incorporation of MT and ST oncoproteins into PP2A heterotrimers is regulated by PP2Ac methylation. PyMT forms wild-type levels of PP2A heterotrimers in the absence of PP2Ac methylation [8,18] but whether SVST or PyST do is not known. We hypothesize that circumventing the normal cellular control of PP2A by PP2Ac methylation is an important strategy for ST- and MT-mediated transformation. Two predictions of this hypothesis are (1) that SVST and PyST will also form PP2A heterotrimers independently of PP2Ac methylation and (2) that down-regulation of PP2Ac methylation to reduce formation of PP2A heterotrimers containing methylation-sensitive B-type subunits will activate progrowth and pro-survival signaling and promote transformation.
Here, we specifically tested whether SVST and PyST form PP2A heterotrimers in the absence of PP2Ac methylation and investigated whether reduction in LCMT-1 promotes transformation in an established human cell transformation system (HEKTERASB56γ cells [23]). We found that SVST and PyST, like PyMT, incorporate into PP2A heterotrimers independently of PP2Ac methylation, suggesting that circumvention of cellular control of PP2A by methylation may be a general strategy for MT and ST oncoproteins. We also found that LCMT-1 knockdown reduces PP2Ac methylation and, consequently, activates Akt and p70/p85 S6K, enhancing anchorage-independent growth. Overall, our findings support the hypothesis that replacement of methylation-sensitive cellular B-type subunits by methylation-insensitive MT and ST oncoproteins is an important strategy in MT- and ST-mediated transformation. Furthermore, the results are consistent with the idea that reduction in PP2Ac methylation may contribute to cancer development and modulation of this methylation may serve as a therapeutic target for cancer.
Materials and Methods
Cell Culture and Creation of Stable Lines Expressing LCMT-1-Directed Small Hairpin RNA
HEKTERASB56γ cells are described elsewhere [23] and were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 20 µM l-glutamine, 100 U/ml penicillin G, 100 µg/ml streptomycin, and 250 ng/ml amphotericin B. To create cells with stable knockdown of LCMT-1 and control cells expressing empty vector, HEKTERASB56γ cells were infected with lentiviruses expressing an LCMT-1 (L3) small hairpin RNA (shRNA) or empty vector (pLKO.1) as previously described [31]. Cells were infected at a multiplicity of infection of 3 to ensure that more than 95% of cells receive at least one viral particle. The new cell lines, HEKTERASB56γ-L3 and HEKTERASB56γ-VC, were expanded and frozen for future experiments. These lines and subsequent lines created from them for this study represent populations of thousands of different clones rather than individual clones.
Creation of the LCMT-1-Rescue Complementary DNA Sequence
To create an LCMT-1-rescue construct that expresses LCMT-1 mRNA resistant to the L3 LCMT-1 shRNA, four silent mutations (depicted here as upper case letters) were introduced by polymerase chain reaction into the LCMT-1 coding region targeted by the L3 LCMT-1 shRNA: 5′ cgtcgaTatgatggaATtC 3′. The entire complementary DNA (cDNA) was sequenced to ensure that only the intended bases were altered.
Creation of HEKTERASB56γ Cells That Stably Express Akt-AA, PME-1, or an shRNA-Resistant LCMT-1
cDNA sequences encoding dominant-interfering Akt-AA (Akt T308A/S473A; gift from Dr Wei Zhou) [32], PME-1, or L3 shRNA-resistant LCMT-1 were cloned into the pLenti6-V5-D-Topo vector using conventional cloning techniques or ligation-independent cloning. Lentiviruses expressing these proteins were then generated using Invitrogen's ViraPower 4-plasmid lentiviral system, and viral titers were determined. HEKTERASB56γ-L3 knockdown cells were infected with the LCMT-1-rescue or Akt-AA lentiviruses and cells stably expressing these constructs (termed L3-rescue and L3-Akt-AA, respectively) were obtained by selecting with 7.5 µg/ml blasticidin S-HCl. Similarly, HEKTERASB56γ vector control (VC) (pLKO.1) cells were infected with the PME-1 lentiviruses to create HEKTERASB56γ-PME-1 OE. For all three of these lines, VC cell lines were created by infection of the same cells with lentiviruses containing an empty pLenti6-V5-D-Topo plasmid.
Antibodies
LCMT-1 was detected using an affinity-purified rabbit anti-LCMT-1 polyclonal antibody, RK3110, described previously [31]. Other antibodies used included sepharose bead-conjugated anti-HA-tag antibody used for immunoprecipitation (F-7 AC; Santa Cruz Biotechnologies, Santa Cruz, CA), anti-HA antibody used for Western blot analysis (16B12; Covance, Princeton, NJ), anti-SVST rabbit polyclonal antibody (gift from W. Hahn), anti-PyST rabbit polyclonal antibody [33], PP2A Bα mouse monoclonal antibody (clone 2G9; Millipore, Bedford, MA), PP2Ac mouse monoclonal antibody (BD Transduction Laboratories, Franklin Lakes, NJ), unmethylated PP2A C subunit mouse monoclonal antibody (clone 1D6 [7]; available from Millipore, Santa Cruz Biotechnology, or request to the corresponding author), p-Akt T308 and p-Akt S473 rabbit monoclonal antibodies (Epitomics, Burlingame, CA), GAPDH mouse monoclonal antibody (Abmart, Shanghai, China), Pme1 mouse monoclonal antibody (B12; Santa Cruz Biotechnology), mouse monoclonal antibodies to p-S6K (p70 T389 and p85 T412) and total rpS6, and rabbit polyclonal antibodies to p-GSK3β S9, p-rpS6 S235/236, total Akt, total p70/p85 S6K, and pAkt substrate ((R/K)X(R/K)XX(pT/pS)) obtained from Cell Signaling Technology (Danvers, MA).
Cell Lysis and Western Analysis
Cells were lysed in an Nonidet P-40-containing lysis buffer (10% glycerol, 20 mM Tris pH 8.0, 137 mM NaCl) as described previously [31]. Samples containing equal amounts of protein as determined by Lowry protein assay (Bio-Rad, Hercules, CA) were analyzed on SDS-polyacrylamide gels. All experiments were conducted using 10% SDS-PAGE gels unless otherwise stated in figure legends. Western blot analysis was performed as described [34], except that secondary antibodies coupled to horseradish peroxidase were used. Bands were visualized by enhanced chemiluminescence and quantitated using a BioRad Fluor-S Max Chemilumimager and Quantity One software. Statistical significance was established by Student's t tests, where P ≤ .05 determines the threshold for significance. *P ≤ .05; **P ≤ .01.
PP2A C Subunit Methylation Assay
The steady-state level of PP2Ac methylation in lysates was determined using a monoclonal antibody specific for unmethylated PP2Ac using our published procedure [8]. Briefly, 20 µl of lysate from VC and L3 cells was treated either with 50 µl of preneutralized base solution (80 mM NaOH, 80 mM HCl, and 200 mM Tris pH 6.8) or with 20 µl of base (200 mM NaOH) to completely demethylate PP2Ac and then neutralized with 30 µl of neutralization buffer (133.3 mM HCl and 333.3 mM Tris pH 6.8) before being analyzed by Western blot analysis for the level of unmethylated PP2Ac and α-tubulin (loading control).
Anchorage-Independent Growth
Anchorage-independent growth was assayed by soft agar analysis as described elsewhere [35,36]. Cells were incubated in agar plates for 3 weeks at 37°C at 10% CO2 and fed with 0.25 ml of 10% FBS in DMEM weekly to maintain moisture and nutrients. Colonies were scored for number and volume using a bench-top microscope (Olympus CK2; Olympus Corp, Center Valley, PA) equipped with an eyepiece micrometer reticle. Colonies 0.1 mm in diameter or larger were counted. All assays were performed three times in triplicate unless otherwise specified in the figure legend. Statistical significance was established by Student's t tests as described above. Small, representative microscopic fields within the dishes were photographed at 40x using an Olympus IX81 microscope (Olympus Corp) equipped with a Nikon Fi1/U2 color camera (Nikon Instruments, Inc, Tokyo, Japan) and processed using NIS Elements software.
Suspension Cultures
To analyze biochemical changes in anchorage-independent cells, cells were plated onto Corning 100-mm ultra-low-attachment culture dishes at 0.5 million cells/plate and grown for 1 week in DMEM supplemented with 10% FBS. The cells formed spherical colonies similar to those seen in soft agar, although, because of the lack of matrix, colonies were not stationary and were sometimes interconnected. Because of this and the fact that attempts to totally dissociate the cells to obtain counts resulted in substantial cell lysis, we used total cell weights as a measure of growth. After 1 week in culture, colonies were collected, washed, and pelleted, and then all excess fluid was removed and the cell pellets were weighed on a highly sensitive scale. Cells were then lysed and analyzed as described previously.
Results
SVST and PyST Do Not Require PP2Ac Methylation for Heterotrimer Formation
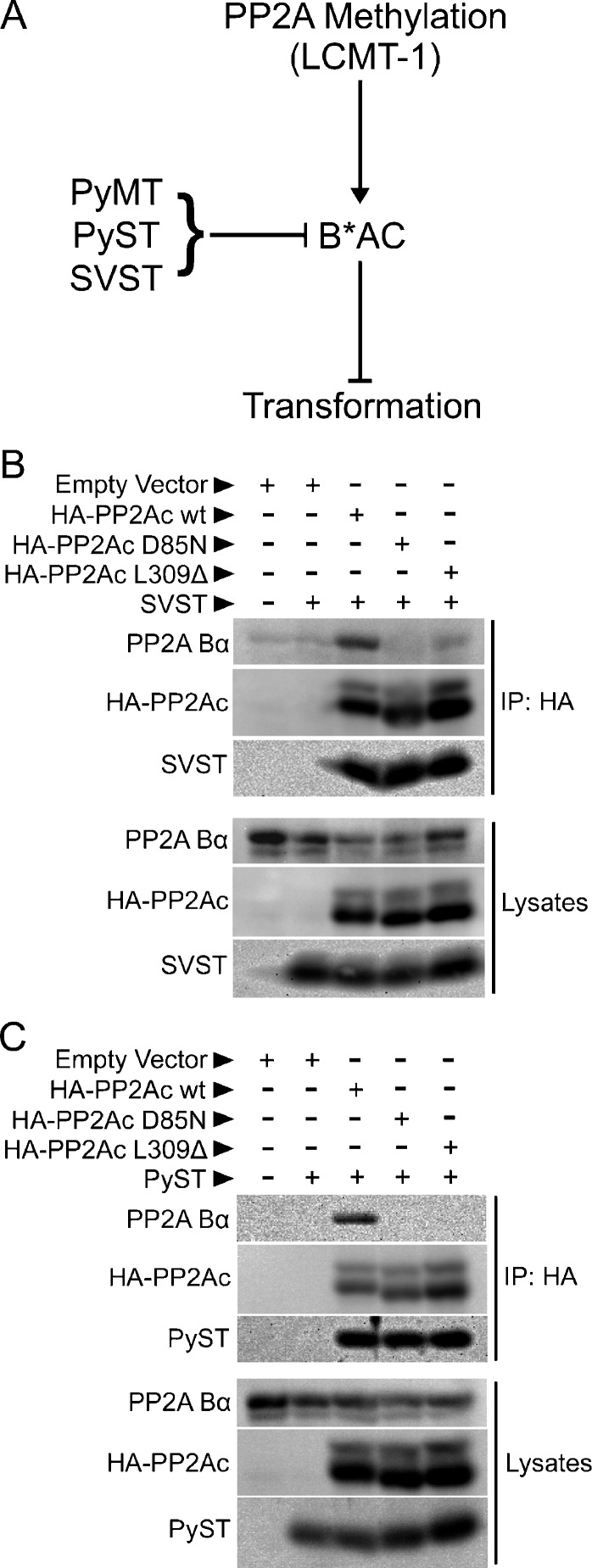
We hypothesized that replacement of methylation-sensitive cellular B-type subunits by methylation-insensitive viral B-type subunits (MT and ST) is an important strategy of polyomavirus and SV40 for circumventing normal control of cell growth and survival during transformation (Figure 1A). To test this hypothesis, we first determined whether SVST and PyST, like PyMT, form PP2A heterotrimers independently of PP2Ac methylation. For these experiments, we used two PP2Ac mutants that had previously been used to distinguish between methylation-sensitive and methylation-insensitive PP2A heterotrimer formation: L309Δ, a PP2Ac mutant lacking the methylated carboxyl-terminal L309, and D85N, an active site PP2Ac mutant with an intact, but unmethylated L309 [8,30]. Of note, the latter mutant indirectly inhibits methylation presumably by blocking association with LCMT-1 (mimicking an LCMT-1 knockdown effect) [37]. We tested whether SVST and PyST could form heterotrimers containing the L309Δ and D85N mutants by using coimmunoprecipitation. SVST and PyST coimmunoprecipitated to the same extent with wild-type, L309Δ, and D85N PP2Ac (Figure 1, B and C), whereas endogenous methylation-sensitive Bα only coimmunoprecipitated with wild-type PP2Ac. This result supports the idea that assembly of PP2A heterotrimers containing MT and ST oncoproteins is not regulated by PP2Ac methylation, whereas formation of at least some of the key PP2A heterotrimers they replace is regulated by the methylation status of PP2Ac.
Figure 1.
Unlike the methylation-dependent cellular B-type subunit, Bα, SVST and PyST can incorporate into PP2A heterotrimers independently of PP2Ac carboxyl methylation. (A) The diagram illustrates a potential strategy of polyomavirus and SV40 in which methylation-insensitive viral B-type subunits (PyMT, PyST, and SVST) specifically replace methylation-sensitive cellular B-type subunits (B*), thus promoting transformation by circumventing normal control of PP2A by methylation. LCMT-1 promotes PP2Ac subunit methylation and the assembly of methylation-sensitive B-type subunits into PP2A heterotrimers (B*AC), which block transformation. Specific targeting of PP2A B*AC complexes by MT and ST oncoproteins promotes transformation. (B and C) HEK 293 cells were cotransfected with empty vector, HA-tagged wild-type PP2Ac (HA-PP2Ac wt), HA-tagged PP2Ac D85N mutant, HA-tagged PP2Ac carboxy-terminal leucine deletion mutant (HA-PP2Ac L309Δ), and SVST (B) or PyST (C) in the combinations indicated. HA-epitope tagged PP2Ac was immunoprecipitated 48 hours later with a sepharose bead-conjugated anti-HA antibody for 1.5 hours at 4°C with rocking to determine the binding of endogenous Bα and SVST (B) or PyST (C). After washing twice with PBS and lysis buffer, immune complexes (upper panels) and lysates (lower panels) were resolved on a 12% SDS-PAGE gel and probed with antibodies to the HA epitope tag, Bα subunit and SVST (B), or PyST (C).
Loss of LCMT-1 Promotes Transformation
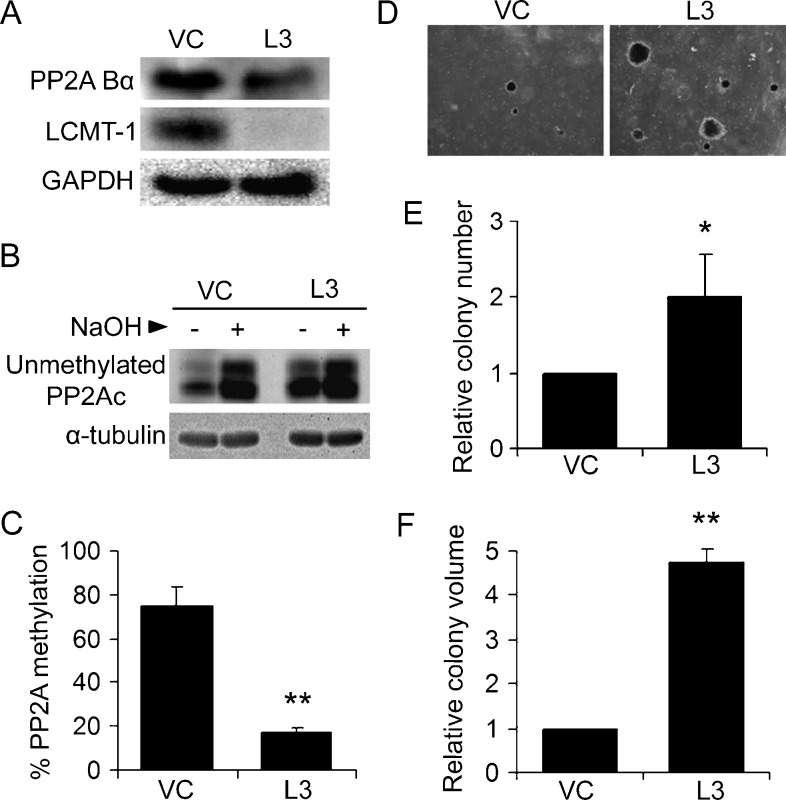
A second prediction of our hypothesis is that reducing PP2Ac methylation by decreasing the level of the methyltransferase responsible for PP2Ac methylation, LCMT-1, would promote transformation. To test this prediction, we used a genetically defined transformation system in which immortalized primary human embryonic kidney cells expressing SV40 large tumor antigen, activated H-Ras, and human telomerase (HEKTER cells [38]) have been weakly transformed by down-regulation of the PP2A B-type subunit, B56γ (HEKTERASB56γ cells [23]). Whereas HEKTER cells can grow indefinitely in culture, they form very few anchorage-independent colonies in soft agar [23,38]. When SVST is expressed in the immortalized HEKTER line, the cells grow robustly in soft agar. Knocking down B56γ, a target of SVST, in HEKTER cells partially recapitulates SVST-mediated transformation phenotype [23]. We chose to use the weakly transformed HEKTERASB56γ line for our studies because it would allow us to determine the cumulative effect of targeting additional methylationsensitive B-type subunits and to detect either increased or decreased transformation on LCMT-1 down-regulation. HEKTERASB56γ cells were infected with vector control (VC) or LCMT-1-directed (L3) shRNA virus, and Western blot analysis was used to verify the knockdown of LCMT-1 (Figure 2A). Quantitation of three independent experiments showed that the LCMT-1 shRNA stably reduced LCMT-1 expression by 80% ± 6% compared with the VC cells. In addition, LCMT-1 knockdown reduced the steady-state level of the methylationsensitive PP2A Bα subunit (Figure 2A) by 28% ± 15% (P =.03), showing that the LCMT-1 knockdown affects at least one B-type subunit that depends on LCMT-1 for heterotrimer formation.
Figure 2.
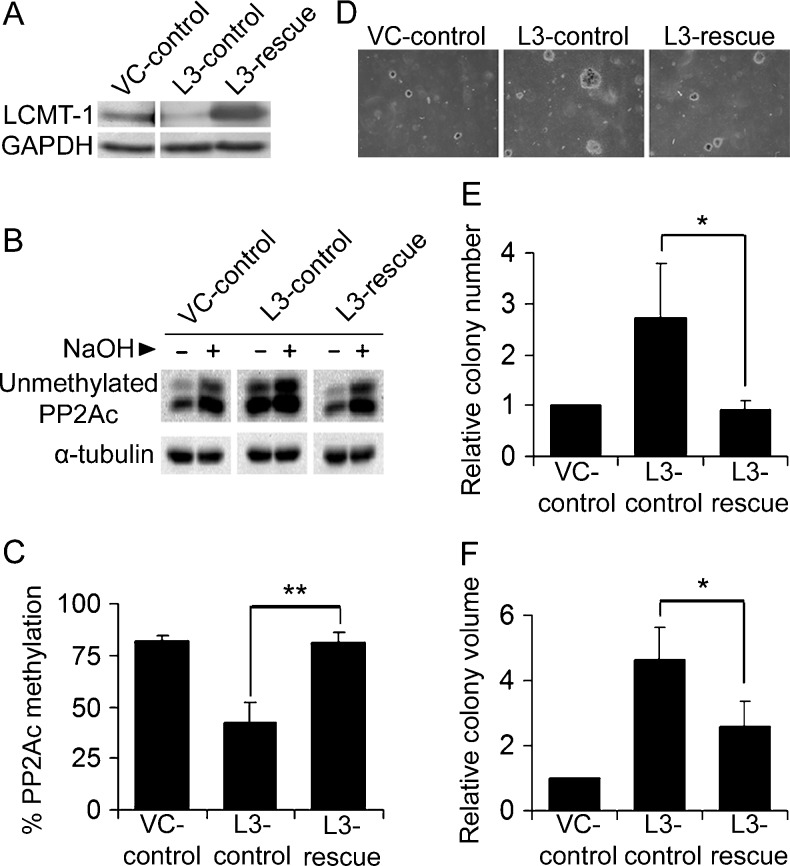
Knocking down LCMT-1 promotes transformation. (A) Knockdown of LCMT-1 in HEKTERASB56γ cells. HEKTERASB56γ cells stably expressing empty pLKO.1 vector control (VC) or LCMT-1 shRNA (L3) were lysed and LCMT-1 and Bα levels were detected by Western blot analysis. GAPDH was used as loading control. (B and C) PP2Ac is highly unmethylated in the LCMT-1 knockdown line. (B) As described in Materials and Methods, equal volumes of lysates from VC and L3 cells were either treated with preneutralized base solution (- lanes; show unmethylated PP2Ac levels in cells) or base treated to completely demethylate PP2Ac and then neutralized (+ lanes; 100% demethylated controls) before being analyzed by Western blot analysis for the level of unmethylated PP2Ac and α-tubulin (loading control). (C) The percent unmethylated PP2Ac was determined by quantitatively comparing the unmethylated PP2Ac signals in the - and + lanes. Percent methylated PP2Ac was calculated by subtracting percent unmethylated PP2Ac from 100. Graph depicts the average percent methylation of PP2Ac in VC and L3 lines. Error bars represent SDs of three independent experiments. (D) Anchorage-independent growth of VC and L3 cells in soft agar. Photographs show small, single, representative fields within the agar wells. Average colony numbers (E) and average colony volumes (F) were determined as described in Materials and Methods, and data are shown in graphs as fold change relative to VC. Error bars represent SD of three independent experiments performed in triplicate. *P ≤ .05. **P ≤ .01.
Next, we measured the effect of LCMT-1 knockdown on the steady-state level of PP2Ac methylation using an assay using a monoclonal antibody specific for unmethylated PP2Ac (Figure 2B). As can be seen by comparing the minus base (-) lanes (which show endogenous levels of unmethylated PP2Ac) in Figure 2B, the amount of unmethylated PP2Ac greatly increased in L3 LCMT-1 knockdown cells compared with the VC control cells. Quantitation of the results showed that only 17% of the total PP2Ac in the LCMT-1 knockdown cells remained methylated compared to 74% methylation in the control cells (Figure 2C). Thus, LCMT-1 knockdown reduced steady-state PP2Ac methylation by more than four-fold.
To examine the role of LCMT-1 and PP2Ac methylation in transformation, VC and L3 cells were evaluated for anchorage-independent growth in soft agar, an assay highly predictive of tumorigenicity [39]. After 3 weeks of growth in agar, colonies were examined by microscopy, revealing that L3 cells formed larger and more abundant colonies (Figure 2D). Quantitative analysis showed that L3 cells had a two-fold increase in colony number (Figure 2E) and more than a four-fold increase in colony volume compared to VC cells (Figure 2F). These results strongly support the hypothesis that LCMT-1 is a negative regulator of transformation and are consistent with the idea that circumvention of PP2Ac methylation-regulated control of PP2A function contributes to MT and ST-mediated transformation.
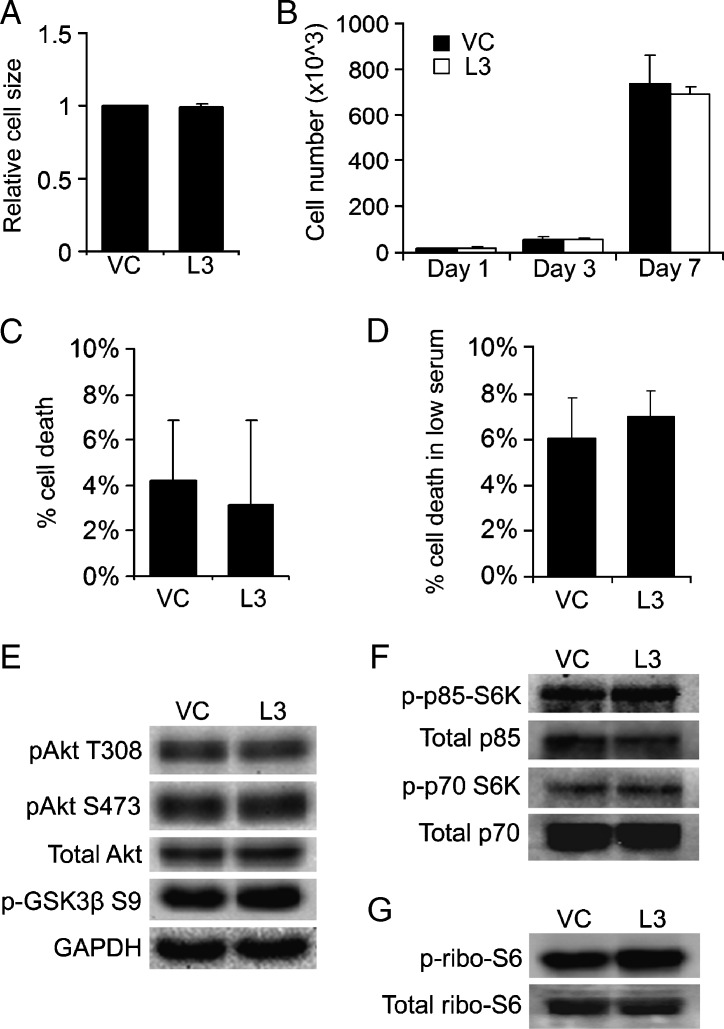
LCMT-1 Knockdown Does Not Affect Cell Size, Proliferation Rate, Survival, or the Akt and p70/p85 S6K Signaling Pathways during Normal Anchorage-Dependent Growth
To begin to dissect how a reduction in LCMT-1 promotes anchorage-independent growth, we tested whether the increase in colony growth of LCMT-1 knockdown cells in soft agar reflects changes in cell size, cell growth, and/or death rates in standard, anchorage-dependent tissue culture. VC and L3 cells growing in anchorage-dependent conditions were analyzed for forward scatter-area (FSC-A) by flow cytometry, which is a function of relative cell size. In three independent experiments, FSC-A was not substantially different (Figure 3A), supporting the conclusion that an increase in cell size on LCMT-1 knockdown does not account for the increase in the number and size of L3 colonies in soft agar. Comparison of the proliferation rates of VC and L3 cells over a 1-week period also showed no significant differences between the two cell lines, indicating that LCMT-1 knockdown does not increase cell proliferation in anchorage-dependent conditions (Figure 3B). Quantitation of the amount of cell death in VC and L3 cultures during adherent cell growth also revealed no significant difference between the cell lines (Figure 3C). Similarly, no significant difference in cell death was seen when the VC and L3 cells were exposed to low serum conditions (0.1% FBS) for 48 hours to stress the cells (Figure 3D). Thus, under both normal and serum-starved growth conditions, no survival advantage is seen for LCMT-1 knockdown cells compared with control cells.
Figure 3.
Adherent control and LCMT-1 knockdown lines show no difference in growth, death, or Akt and S6K signaling. (A) Non-confluent VC and LCMT-1 knockdown (L3) cells growing on 10-cm tissue culture dishes for 48 hours were trypsinized, washed, and dispersed into single-cell suspensions. About 20,000 cells/sample were analyzed using an AccuriC6 Flow Cytometer (BD Bioscience, Franklin Lakes, NJ) to compare cell size as determined by FSC-A. The average cell size is presented in the graph as fold change relative to VC. (B) Growth rates of adherent VC and L3 lines were assessed by plating 10,000 cells on 10-cm dishes on day 0 and collecting and counting all viable cells (adherent and detached) at days 1, 3, and 7. Cells were stained with Trypan blue to exclude dead cells and counted with a hemacytometer. Graph represents the average number of live cells at each time point. (C) The VC and L3 lines were plated at 50,000 cells per 3.5-cm well and grown for 72 hours. Both adherent and detached cells were collected, stained with Trypan blue to identify dead cells, and counted with a hemacytometer. The average percentage of dead cells of the VC and L3 lines is presented in the graph as percent cell death. (D) VC and L3 cell lines were plated at 50,000 cells/well in triplicate in a six-well dish and cultured in DMEM supplemented with 10% FBS for 24 hours. Cells were then serum-starved (0.1% FBS) for 48 hours, and the percentage of dead cells was determined. The average percentage of dead cells in each line is presented in the graph. (E–G) Lysates from adherent VC and L3 cells were analyzed by Western blot analysis for pAkt T308, pAkt S473, pGSK3β S9, p-p85 S6K, p-p70 S6K and p-rpS6. GAPDH, total Akt, total p70 and p85 S6K, and total rpS6 were used as loading controls. No statistically significant changes were seen in signaling in three independent experiments. Error bars in all panels represent SD of three experiments. *P ≤ .05. **P ≤ .01.
To assess whether LCMT-1 knockdown activates progrowth/prosurvival signaling pathways during anchorage-dependent growth, we compared the levels of regulatory phosphorylations on Akt, the Akt substrate, GSK3β, p70/p85 S6K, and ribosomal protein S6 (rpS6) in adherent cells. No significant differences in these phosphorylations were detected between the VC and L3 cells (Figure 3, E–G). These results indicate that the activation state of these signaling molecules is not altered by LCMT-1 knockdown in adherent cultures, consistent with the lack of effect of LCMT-1 knockdown on proliferation and survival during normal anchorage-dependent growth.
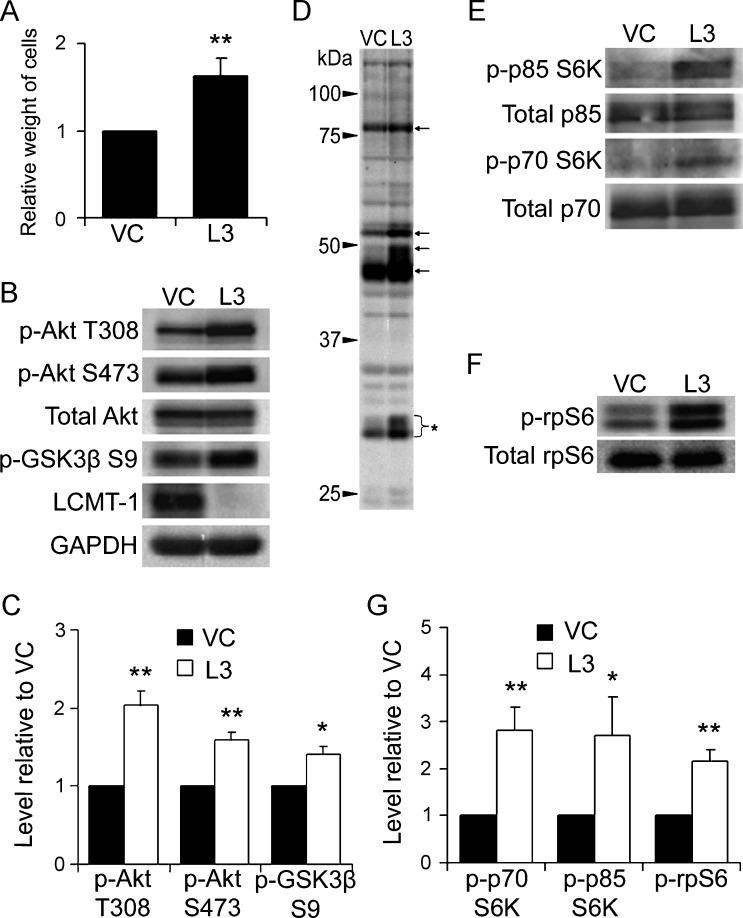
LCMT-1 Knockdown Activates the Akt and p70/p85 S6K Pathways in Anchorage-Independent Conditions
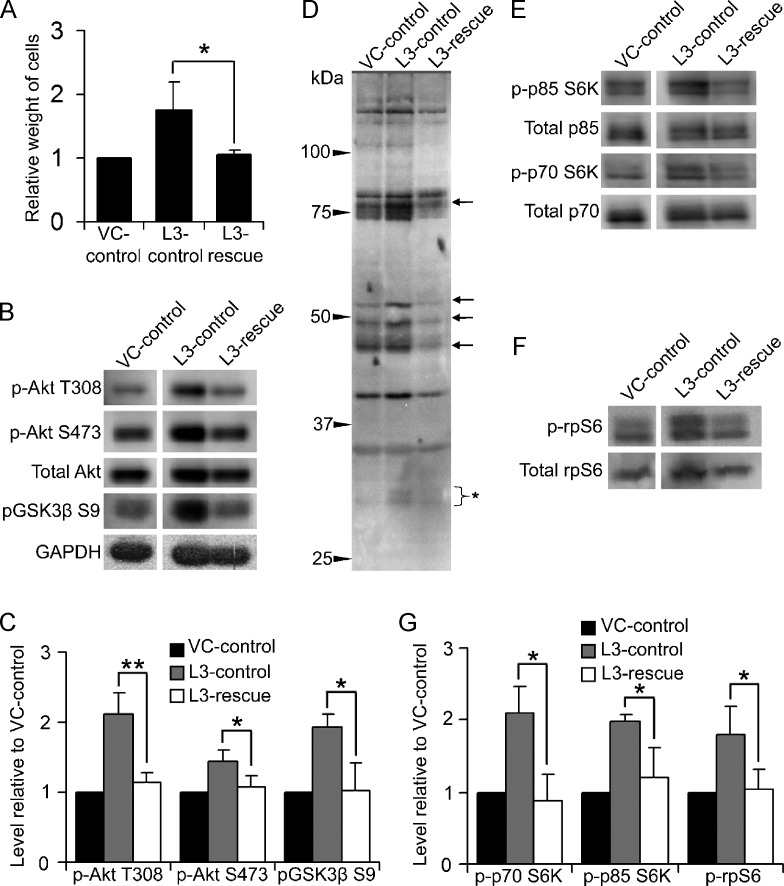
Our observation that LCMT-1 knockdown increased colony number and size in soft agar but had no significant effect on growth or survival in anchorage-dependent conditions raised the intriguing possibility that LCMT-1 knockdown might enhance growth and survival signaling exclusively during anchorage-independent cell growth. When anchorage-dependent cells are unable to attach to an appropriate substratum, reduced growth and survival signaling normally occurs, resulting in cessation of cell proliferation and eventually cell death [40]. We hypothesized that LCMT-1 is necessary for the down-regulation of key proliferation and survival signals in anchorage-independent conditions and that LCMT-1 knockdown inappropriately maintains elevated levels of progrowth and prosurvival signaling during anchorage-independent growth. To test this hypothesis, we analyzed the Akt and p70/p85 S6K signaling pathways in VC and L3 cells growing in anchorage-independent conditions on ultra-low-adherence tissue culture dishes. In these dishes, both the VC and L3 cell lines proliferate and form spherical, anchorage-independent clusters of cells. Similar to results from soft agar growth, the LCMT-1 knockdown cells showed a selective advantage over the control cells as indicated by a 70% increased total weight of the L3 cells compared with the VC cells after 1 week of growth under these conditions (Figure 4A). Considering the difference in assay time, we would expect that the L3 suspension colonies would have had a similar growth differential to the VC line as seen in soft agar if they were grown for 3 weeks in suspension cultures. Western blot analysis of the VC and L3 cell suspension cultures revealed a number of pro-growth and prosurvival signaling differences. Relative to VC cells, L3 cells have increased activated Akt as indicated by increased phosphorylation at both T308, which is located in the kinase domain, and S473, which is located in the C-terminal regulatory domain (Figure 4, B and C). Phosphorylation of these two sites is carried out by different kinases, and while phosphorylation of T308 partially activates Akt, full activation requires phosphorylation at both sites [41]. Furthermore, a downstream target of the Akt cell growth and survival pathway, GSK3β, showed significantly increased phosphorylation on Ser9 in L3 cells (Figure 4, B and C). Phosphorylation of Ser9 on GSK3β is inhibitory and would thus be expected to reduce the reported proapoptotic activities of GSK3β [42]. To further examine activation of Akt, we probed lysates from these cells with a phospho-Akt substrate antibody that specifically recognizes the (R/K)X(R/K)XX(pT/pS) motif when it is phosphorylated on the serine/threonine residue. Consistent with our finding of increased activated Akt on LCMT-1 knockdown, this antibody detected substantial increases in the phosphorylation of a number of proteins in L3 cells compared to VC cells (Figure 4D).
Figure 4.
LCMT-1 knockdown activates Akt and S6K signaling in anchorage-independent conditions. (A) Equal numbers of VC and L3 cells were seeded on low-binding tissue culture dishes to analyze differences during anchorage-independent growth. After 1 week, suspension cells were weighed to assess growth. In the graph, data are presented as average fold change relative to VC for three independent experiments. (B) Lysates from VC and L3 suspension cells were analyzed by Western blot analysis for changes in activation of Akt. GAPDH and total Akt were used as loading controls. Western blot analysis for LCMT-1 confirmed the knockdown of LCMT-1 in suspension cultures. (C) Graph depicts the average levels of phospho-Akt T308, phospho-Akt S473, and phospho-GSK3β S9 in three independent immunoblot experiments as fold change relative to VC. (D) An anti-Akt phospho-substrate motif (R/K)X(R/K)XX(pT/pS) antibody was used to probe the VC and L3 suspension cell lysates. Arrows highlight some proteins with increased phosphorylation in the L3 cells relative to VC. Bracket with asterisk indicates phospho-rpS6, which is known to cross-react with this antibody. (E and F) Lysates were probed for changes in p70 and p85 S6K activation and rpS6 phosphorylation. Total p70 and p85 S6K and total rpS6 were used as loading controls. (G) Graph represents average fold change in the levels of phospho-p85, phospho-p70 and phospho-rpS6 in three independent immunoblot experiments relative to VC. Experiments were performed in triplicate and error bars in all panels represent SD. *P ≤ .05. **P ≤ .01.
The (R/K)X(R/K)XX(pT/pS) substrate phosphorylation motif is also shared with S6K; therefore, some of the proteins with increased phosphorylation in the L3 lane of Figure 4D could be p70/p85 S6K substrates. For example, the bracketed protein of ∼30 kDa in Figure 4D is likely rpS6, which is a substrate for p70 S6K but not Akt. Probing for activating phosphorylations on p70/p85 S6K showed that these kinases are indeed activated by LCMT-1 knockdown (Figure 4, E and G). Immunoblot analysis with phospho-rpS6 and total rpS6 antibodies also confirmed that LCMT-1 knockdown increases rpS6 phosphorylation on a site that correlates with increased protein translation [43,44] (Figure 4, F and G). These results support the idea that LCMT-1 knockdown increases survival in an anchorage-independent environment through modulation of the Akt and p70/p85 S6K pathways.
Effects of LCMT-1 Knockdown on Growth and Signaling Are Rescued by Exogenous Expression of LCMT-1
To confirm that the effects of enhanced transformation and altered signaling in the LCMT-1 knockdown line are specifically due to decreased LCMT-1, we tested whether the effects of LCMT-1 knockdown could be rescued by exogenously expressing LCMT-1. To accomplish this, the original VC cells were infected with an empty expression vector (creating VC-control cells) and the original LCMT-1 knockdown cells (L3) were infected with either an empty vector (creating L3-control cells) or a vector expressing an L3 shRNA-resistant LCMT-1 mRNA (creating L3-rescue cells). Western blot analysis verified that LCMT-1 remained knocked down in the L3-control line and that LCMT-1 protein expression was rescued in the L3-rescue line (Figure 5A). Analysis of the level of PP2Ac methylation in these cell lines demonstrated that restoration of LCMT-1 expression restored the normal level of PP2Ac methylation in the L3-rescue cells (Figure 5, B and C). To examine the ability of exogenous LCMT-1 expression to rescue the effect of LCMT-1 knockdown on transformation, VC-control, L3-control, and L3-rescue lines were subjected to soft agar analysis. The number of colonies produced by the L3-rescue cells was reduced almost three-fold from the L3-control cells and was comparable to the VC-control cells (Figure 5, D and E), indicating that loss of LCMT-1 was indeed responsible for the increase in colony number observed on LCMT-1 knockdown. LCMT-1 reexpression (L3-rescue) also significantly reversed colony size compared to the LCMT-1 knockdown cells (L3-control; Figure 5, D and F). These results support the conclusion that reduction of LCMT-1 enhances transformation in the HEKTERASB56γ cell line, presumably by hampering PP2A's tumor suppressor function. Consistent with the hypothesis that LCMT-1 has a tumor suppressor function, overexpression (OE) of LCMT-1 in HEKTERASB56γ VC cells also substantially reduced both colony number and volume in soft agar (data not shown). Together, these data argue that loss of PP2A methylation promotes transformation, whereas increasing PP2A methylation reduces transformation.
Figure 5.
Effects of LCMT-1 knockdown on transformation are rescued by expression of an shRNA-resistant LCMT-1. (A) Rescue of the LCMT-1 knockdown line. Lysates from VC and L3 cells stably expressing an empty control plasmid (VC-control and L3-control, respectively) and L3 LCMT-1 knockdown cells stably expressing an LCMT-1 rescue plasmid (L3-rescue) were analyzed by Western blot analysis for LCMT-1 protein expression. (B and C) PP2Ac methylation is rescued in the L3 rescue cell line. (B) Equal volumes of lysates from VC-control, L3-control, and L3-rescue were either treated with preneutralized base solution (- lanes; show unmethylated PP2Ac levels in cells) or base treated and then neutralized (+ lanes; 100% demethylated controls) before being analyzed by Western blot analysis for the level of unmethylated PP2Ac and α-tubulin (loading control). (C) Graph depicts the average percent methylation of PP2Ac in the lysates, calculated as described in the legend to Figure 2C. (D) Anchorage-independent growth of VC-control, L3-control, and L3-rescue cells in soft agar. Photographs show small, single, representative fields within the agars. Average colony numbers (E) and average colony volumes (F) were determined, and data are shown in graphs as fold change relative to VC-control. Error bars in all panels represent SD of three independent experiments. *P ≤ .05. **P ≤ .01.
To determine whether reexpression of LCMT-1 in L3 cells reverses the changes in the Akt and p70/p85 S6K signaling pathways induced by LCMT-1 knockdown, the VC-control, L3-control, and L3-rescue cells were grown in suspension in ultra-low-adherent culture dishes. Reexpression of LCMT-1 completely reversed the anchorage-independent growth advantage observed in L3-rescue cells (Figure 6A). Correspondingly, the phosphorylation levels of T308 and S473 on Akt and of S9 on GSK3β were fully reversed to control levels (Figure 6, B and C; compare L3 rescue to VC-control). Levels of phosphorylation on (R/K)X(R/K) XX(pT/pS) motif-containing proteins were also reversed to control levels (Figure 6D). Lastly, Western blot analysis revealed that reexpression of LCMT-1 fully reversed the activation of p70/p85 S6K and rpS6 phosphorylation (Figure 6, E–G). These results demonstrate that the signaling changes detected in this system upon LCMT-1 knockdown are due specifically to reduction in the LCMT-1 protein level. They also support the hypothesis that that loss of PP2A methylation promotes transformation, although we cannot rule out a possible contribution of a yet to be discovered LCMT-1 target.
Figure 6.
Effects of LCMT-1 knockdown on biochemical signaling are rescued by reexpression of LCMT-1. (A) Normalization of anchorage-independent growth by LCMT-1 reexpression. Anchorage-independent growth of VC-control, L3-control, and L3-rescue cells (defined in Figure 5A) were assessed as in Figure 4A by weighing cells after 1 week in suspension culture. Data are presented as fold-change relative to VC. (B, C, and D) Normalization of Akt signaling by LCMT-1 reexpression. (B) Lysates from VC-control, L3-control, and L3-rescue suspension cells were analyzed by Western blot analysis for changes in activation of Akt. GAPDH and total Akt were used as loading controls. (C) Graph depicts the average fold change in the levels of phospho-Akt T308, phospho-Akt S473, and phospho-GSK3β S9 in three immunoblot experiments relative to VC-control. (D) Akt phospho-substrate antibody was used to probe lysates from the VC-control, L3-control, and L3-rescue lines. Arrows indicate some proteins whose phosphorylation increased on L3 knockdown but returned to control levels on reexpression of LCMT-1. Bracket indicates phospho-rpS6. (E and F) Rescue of S6K activation and rpS6 phosphorylation by LCMT-1 reexpression. Lysates were probed for changes in the activation state of p70 and p85 S6K and rpS6 phosphorylation. Total p70 and p85 S6K and total rpS6 were used as loading controls. (G) Graph represents average fold change in the levels of phospho-p85, phospho-p70, and phosphorpS6 in three immunoblot experiments relative to VC-control. Error bars in all panels represent SD of three independent experiments. *P ≤ .05. **P ≤ .01.
Akt Activation Is Necessary for the Enhanced Transformation Caused by LCMT-1 Knockdown
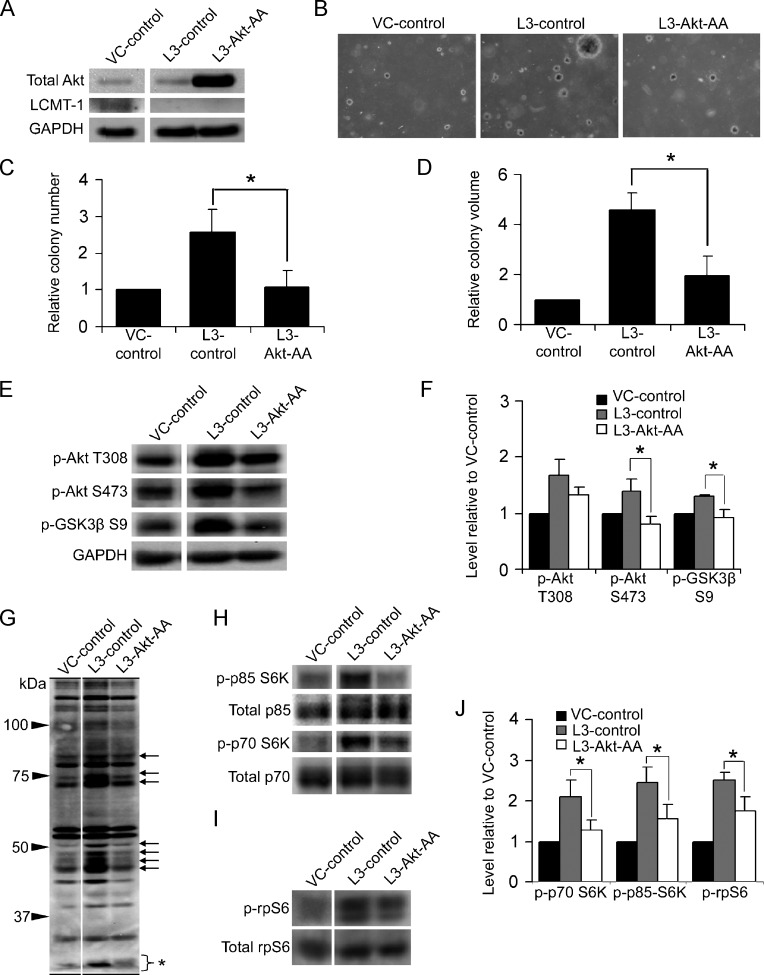
To test the hypothesis that LCMT-1 knockdown increases transformation through activating the Akt survival pathway, we expressed a dominant-negative Akt (Akt-AA) in LCMT-1 (L3) knockdown cells (creating L3-Akt-AA cells). VC-control, L3-control, and L3-Akt-AA cell lines were analyzed by Western blot to confirm the expression of the mutant Akt construct and the maintenance of LCMT-1 knockdown in L3-Akt-AA cells (Figure 7A). These lines were then subjected to soft agar analysis to assay for anchorage-independent growth. The expression of Akt-AA completely reversed the increase in colony number (∼2.4-fold) observed upon LCMT-1 knockdown and reduced the average colony volume of LCMT-1 knockdown cells by more than 2.3-fold (Figure 7, B–D). In contrast, the expression of Akt-AA in HEKTERASB56γ VC cells resulted in only a ∼30% reduction in soft agar colony number that was not statistically significant and reduced colony size by only 6% in soft agar (data not shown), indicating that the effect of expressing the kinase-deficient Akt in L3 knockdown cells is largely specific for LCMT-1.
Figure 7.
Akt activation is necessary for the enhanced transformation caused by LCMT-1 knockdown. (A) Lysates from VC and L3 cells stably expressing an empty control plasmid (VC-control and L3-control, respectively) and lysates from L3 cells stably expressing dominant-negative Akt-AA (L3-Akt-AA) were analyzed by Western blot analysis for the expression of Akt-AA, LCMT-1, and GAPDH. (B) knockdownknockdownAnchorage-independent growth of VC-control, L3-control, and L3-Akt-AA cells in soft agar. Photographs show small, single, representative fields within the agars. Average colony numbers (C) and average colony volumes (D) were determined, and data are shown in graphs as fold change relative to VC-control. Error bars represent SD of three independent experiments performed in triplicate. (E) Lysates from VC-control, L3-control, and L3-Akt-AA suspension colonies were analyzed by Western blot analysis for changes in activation of endogenous Akt. GAPDH was used as a loading control. (F) Graph depicts the average fold change in the levels of phospho-Akt T308, phospho-Akt S473, and phospho-GSK3β S9 in three immunoblot experiments relative to VC-control. (G) Probing of VC-control, L3-control, and L3-Akt-AA lysates with phospho-Akt substrate antibody shows that dominant-negative Akt expression prevents the increased phosphorylation of many proteins caused by LCMT-1 knockdown (arrows show examples). The bracket with asterisk indicates phospho-rpS6, which ran at the bottom of this 7.5% SDS-PAGE gel. (H and I) Lysates were probed for changes in p70 and p85 S6K activation and rpS6 phosphorylation. Total p70 and p85 S6K and total rpS6 were used as controls. (J) Graph depicts the average fold change in the levels of phospho-p85, phospho-p70, and phospho-rpS6 in three immunoblot experiments relative to VC-control. Error bars represent SD of three independent experiments. For all graphs: *P ≤ .05. **P ≤ .01.
To examine the effect of dominant-negative Akt-AA expression on Akt signaling, VC-control, L3-control, and L3-Akt-AA cells were grown in suspension and analyzed by Western blot. Akt S473 phosphorylation and GSK3β S9 phosphorylation were reduced to control levels, whereas Akt T308 phosphorylation was partially reduced but not significantly (Figure 7, E and F). Immunoblot analysis with the (R/K)X(R/K)XX(pT/pS) motif antibody showed that phosphorylation of most proteins affected by LCMT-1 knockdown was also reversed to control levels (Figure 7G). Lastly, we probed lysates from these lines for p70/p85 S6K activating phosphorylations and phosphorylation of rpS6. p70/p85 S6K activating phosphorylations were substantially and significantly reduced in L3-Akt-AA cells as was rpS6 phosphorylation (Figure 7, H–J). Together, these results show that Akt signaling is required for the enhanced transformation caused by LCMT-1 knockdown and for much of the p70/p85 S6K pathway activation.
Overexpression of the PP2Ac Methylesterase, PME-1, Causes Similar Changes in Anchorage-Independent Growth and Signaling As LCMT-1 Knockdown
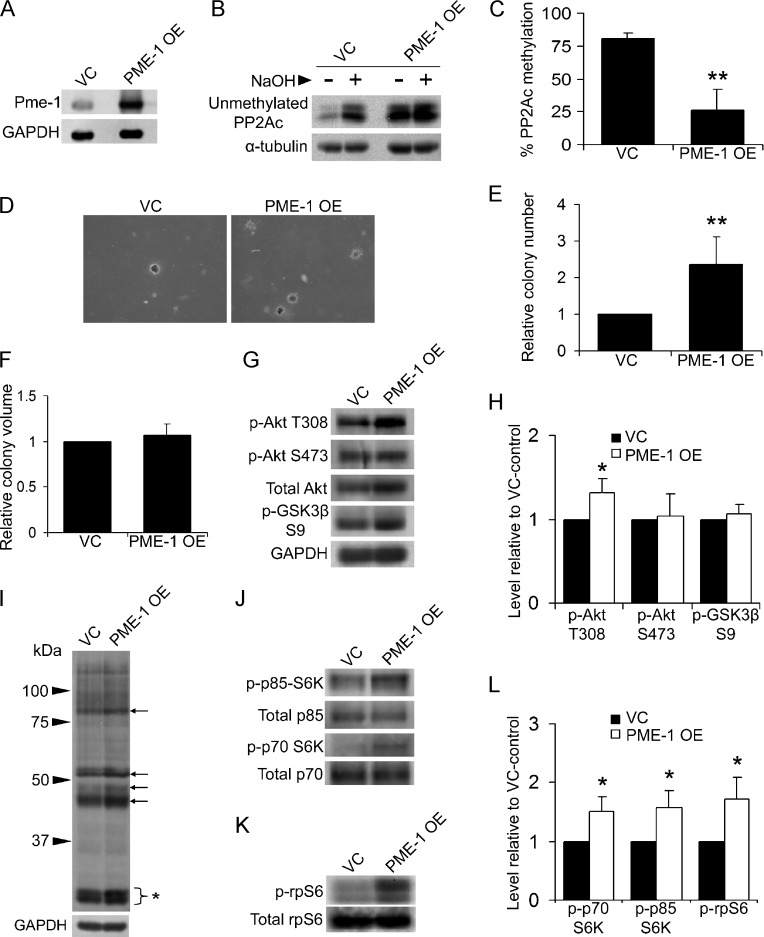
PME-1 overexpression provides another approach to test the effects of reduced PP2A methylation on transformation. To determine whether PME-1 overexpression would cause the same effects as LCMT-1 knockdown, we overexpressed PME-1 in HEKTER-ASB56γ cells (creating PME-1 OE cells). VC and PME-1 OE cells were analyzed by Western blot analysis to verify PME-1 expression (Figure 8A). PP2Ac methylation in cell lysates was reduced from 81% for VC cells to 26% for PME-1 OE cells (Figure 8, B and C). Results of experiments evaluating anchorage-independent growth in soft agar showed that PME-1 overexpression significantly increases colony number, but not colony volume (Figure 8, D–F). Western blot analysis of VC and PME-1 OE suspension cell lysates for changes in the Akt and p70/p85 S6K pathways showed that PME-1 overexpression significantly increased T308 phosphorylation on Akt but not S473 phosphorylation on Akt or pGSK3β S9 phosphorylation (Figure 8, G and H). Probing lysates from VC and PME-1 OE cells with (R/K)X(R/K)XX(pT/pS) substrate motif antibody demonstrated that PME-1 overexpression increased the phosphorylation of several proteins migrating at positions of proteins whose phosphorylation was increased in LCMT-1 knockdown cells (compare Figure 8I to Figure 4D). Lastly, activating phosphorylation of p70/p85 S6K and phosphorylation of rpS6 were significantly increased (Figure 8, J–L). These results show that overexpression of PME-1 reduces PP2Ac methylation, increases transformation, and alters Akt and p70/p85 S6K signaling in a manner similar to LCMT-1 knockdown.
Figure 8.
Overexpression of the PP2A methylesterase, PME-1, enhances transformation, and elicits similar biochemical changes as LCMT-1 suppression. (A) Lysates from HEKTERASB56γ cells stably expressing either an empty vector (VC) or excess PME-1 (PME-1 OE) were analyzed by Western blot analysis for the expression of PME-1. (B and C) PP2Ac methylation is reduced in the PME-1 OE cell line. (B) Equal volumes of lysates from VC and PME-1 OE lines were either treated with preneutralized base solution (- lanes; show unmethylated PP2Ac levels in cells) or base treated and then neutralized (+ lanes; 100% demethylated controls) before being analyzed by Western blot analysis for the level of unmethylated PP2Ac and α-tubulin (loading control). (C) Graph depicts the average percent methylation of PP2Ac in the lysates, calculated as described in the legend to Figure 2C. Error bars represent SD of three independent experiments. (D) Anchorage-independent growth of VC and PME-1 OE cells in soft agar. Photographs show small, single, representative fields within the agars. Average colony numbers (E) and average colony volumes (F) were determined, and data are shown in graphs as fold change relative to VC. Error bars represent SD of six independent experiments performed in triplicate. (G) Lysates from VC and PME-1 OE suspension cells were analyzed by Western blot analysis for changes in activation of Akt. GAPDH was used as a loading control. (H) Graph depicts the average fold change in the levels of phospho-Akt T308, phospho-Akt S473, and phospho-GSK3β S9 in three independent immunoblot experiments relative to VC. (I) Akt phospho-substrate antibody was used to probe the VC and PME-1 OE lysates. Arrows highlight some proteins whose phosphorylation increased in PME-1 OE cells. Bracket with asterisk indicates phospho-rpS6. (J and K) Lysates were probed for changes in p70 and p85 S6K activation and rpS6 phosphorylation. Total p70 and p85 S6K and total rpS6 were used as controls. (L) Graph represents the average fold change in the levels of phospho-p85, phospho-p70, and phospho-rpS6 in three immunoblot experiments relative to VC. Error bars represent SD of three independent experiments. For all graphs: *P ≤ .05. **P ≤ .01.
Discussion
Our results demonstrate that SVST and PyST, like PyMT, form heterotrimers with PP2A in a methylation-insensitive manner. In addition, reducing PP2Ac methylation by knocking down LCMT-1 or overexpressing PME-1 enhances progrowth and prosurvival signaling, promoting transformation in the genetically defined HEKTERASB56γ human cell system. Overall, these results lead us to propose a model for MT and ST oncoprotein-mediated transformation in which methylation-insensitive viral B-type subunits (MT and ST) replace methylation-sensitive cellular PP2A B-type subunits to circumvent the antigrowth, antiproliferative effects of methylation-sensitive PP2A heterotrimers (Figure 9). The fact that both polyomavirus and SV40 oncoproteins form PP2A heterotrimers independent of the methylation status of PP2Ac suggests that circumventing control by PP2Ac methylation is a general strategy for MT and ST-mediated transformation. Together, these results provide new insight into the mechanism of transformation by MT and ST oncoproteins and are consistent with the idea that a reduction in LCMT-1 amount or activity could contribute to human cancer.
Figure 9.
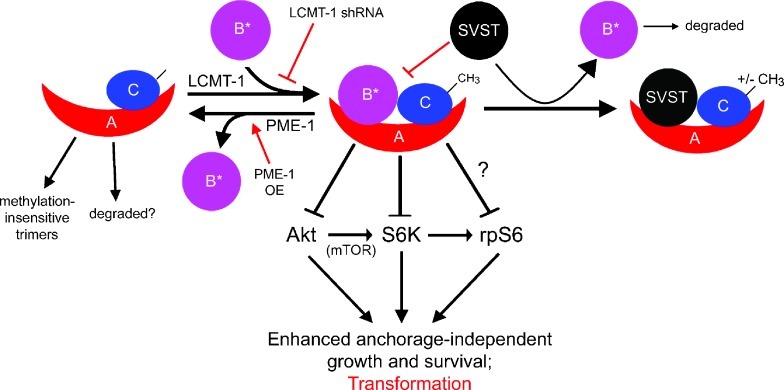
Proposed model for the negative regulation of anchorage-independent growth and survival by methylation-sensitive PP2A heterotrimers and the protransformation effects of reducing these heterotrimers by SVST expression, LCMT-1 knockdown, or PME-1 overexpression. Methylation of PP2Ac through LCMT-1 promotes incorporation of methylation-sensitive B-type subunits (B*) into heterotrimers that dephosphorylate and thereby inactivate Akt and S6K. PP2A methylation-sensitive heterotrimers can act on S6K in both an Akt-dependent and independent manner to block downstream survival and translation signals and thus transformation. PP2A methylation-sensitive heterotrimers also promote the dephosphorylation of rpS6 independently of S6K to block translation, although it is not known if this is a direct or indirect effect. Methylation-insensitive SVST (or PyST and PyMT) oncoprotein replaces methylation-sensitive B-type subunits, promoting transformation by preventing the dephosphorylation and inactivation of Akt and S6K. Transformation is also enhanced by reducing methylation-sensitive PP2A heterotrimers by inhibition of LCMT-1 (e.g., through shRNA or mutation) or PME-1 overexpression (PME-OE). For simplicity, only the targets of methylation-sensitive PP2A heterotrimers examined in this study are shown in this schematic.
LCMT-1 knockdown in HEKTERASB56γ cells increased both the number and size of the colonies observed in soft agar assays, indicating that loss of LCMT-1 causes additional signaling changes to those already present in the HEKTERASB56γ cells. Activation of Akt, which was demonstrated by both direct measurement of activating phosphorylations and analysis of downstream signaling, was required for LCMT-1 knockdown-induced transformation. Phosphorylation of Akt T308 was particularly increased on LCMT-1 knockdown, suggesting that this phosphorylation site may be regulated directly or indirectly by one or more methylation-sensitive PP2A B-type subunits. Consistent with this possibility, the highly methylation-sensitive PP2A Bα subunit has been reported to direct the dephosphorylation of Akt T308 [13]. Another possible candidate is B′β, which has been reported to negatively regulate Akt T308 phosphorylation and seems to be methylation-sensitive [12,15]. Akt S473 phosphorylation, however, is regulated by PP2A B′γ (B56γ) [14], which is knocked down already in the HEKTERASB56γ cells. LCMT-1 knockdown further increases phosphorylation at this site, suggesting that LCMT-1 may regulate additional PP2A heterotrimeric forms that target Akt S473. Because B′β has also been reported to promote dephosphorylation of Akt S473 [15], it is a possible target for LCMT-1-mediated effects. Bα and B′α, which are both highly methylation-sensitive [8,12], do not seem to be candidates for modulation of Akt S473 phosphorylation because Bα or B′α knockdown does not affect phosphorylation on this site [13,14].
LCMT-1 knockdown also activated p70/p85 S6K and increased phosphorylation of their downstream target, rpS6, on a site that correlates with increased translation [43,44]. Given that S6K can be activated by Akt through the mTOR pathway [45,46], much of the LCMT-1-dependent activation of the S6K pathway might be a consequence of Akt activation. Consistent with this possibility, dominant-negative Akt expression greatly reduced p70/p85 S6K activation observed on LCMT-1 knockdown. However, the small amount of residual phosphorylation on p85 S6K (P = .04 when compared with control) and more substantial residual phosphorylation of rpS6 (P = .02 when compared with control) suggest there is also more direct, Akt-independent regulation of this pathway by methylationsensitive PP2A heterotrimers. Consistent with this idea, Bα-containing PP2A heterotrimers have been reported to directly bind to and negatively regulate p70 S6K [16]. Furthermore, E4orf4, an adenovirus protein that inhibits the Bα-containing PP2A heterotrimers, increases the level of activated p70 S6K and inhibitory phosphorylation of 4EBP1 [47], therefore likely promoting translation. Thus, whereas Akt-dependent signaling is required for LCMT-1 knockdown to enhance transformation in HEKTERASB56γ cells, methylation-sensitive PP2A heterotrimers regulate translation in both Akt-dependent and independent manners.
Interestingly, the importance of LCMT-1 for the regulation of Akt and p70/p85 S6K and increased growth and/or survival was revealed in the current study only under anchorage-independent growth conditions. This finding is significant considering that anchorage-independent growth facilitated by resisting anoikis, programmed cell death induced by detachment of cells from their extracellular matrix (ECM), is a critical step in tumorigenesis [40]. Under anchorage-dependent conditions, growth and survival signaling are normally initiated by the interaction of integrins with the ECM. When cells detach from the substratum, these signals are abrogated, and cells undergo growth arrest and anoikis. Cancer cells resist anoikis by aberrant ECM-independent growth and survival signaling and can proliferate and survive in anchorage-independent conditions. Inhibition of methylation-sensitive PP2A heterotrimers by LCMT-1 knockdown may provide prosurvival and progrowth signaling normally initiated by the interaction of integrins with the ECM. Consistent with this idea, PP2A is involved in integrin-mediated growth and survival signaling [29,48–54]. Also, Src, a kinase that phosphorylates and inactivates PP2A [55], functions in prosurvival integrin signaling [40]. Importantly, inhibition of PP2A is known to cause increased activation of Akt and p70 S6K [47,53,56,57]. Therefore, we propose that LCMT-1 knockdown promotes anchorage-independent growth by reducing methylation-sensitive PP2A complexes that normally function to promote growth arrest and anoikis through inactivation of Akt under anchorage-independent conditions. Because SVST targets methylation-sensitive B-type subunits for replacement and evades control of heterotrimer formation by PP2Ac methylation (Figure 1), the ability of SVST expression to induce HEKTER cells to grow in an anchorage-independent environment may be explained in part by these findings.
In contrast to previous results with HeLa cells where LCMT-1 knockdown induced apoptosis in a small portion of the cells [12,31], no increase in cell death on LCMT-1 knockdown was seen in the current study. The cell death in HeLa cells was reported to be due at least in part to mitotic checkpoint errors [31]. Therefore, it would not be unreasonable to expect that, with strong prosurvival signaling, these errors might not lead to death. Thus, differences in prosurvival signaling between HeLa and HEKTERASB56γ may explain this disparity. Consistent with this possibility, we have found that LCMT-1 knockdown increases apoptosis in some cancer cell lines (e.g., HeLa and H1299 cells) but not others.
PME-1 overexpression also reduced PP2Ac methylation, enhanced transformation, and caused many of the same signaling effects as LCMT-1 knockdown, further supporting the hypothesis that reduction in PP2Ac methylation enhances transformation through up-regulation of Akt and p70/p85 S6K pathways. The reduced potency of overexpressing PME-1 compared to LCMT-1 knockdown may result from PME-1 overexpression having other effects on PP2A holoenzyme assembly or activity. This possibility is consistent with the report that PME-1 functions in PP2A biogenesis [2,58], and with the finding that PME-1 knockout in mice results in reduced PP2A activity toward a phosphopeptide substrate and reduced total PP2A protein in several tissues [59]. Consistent with our findings that PME-1 over-expression promotes transformation, PME-1 was found to be over-expressed in human gliomas and knockdown of PME-1 in HeLa cells reduced transformation [60]. Together, these data argue strongly that loss of PP2A methylation promotes transformation.
Consistent with the idea that MT and ST oncoproteins transform cells in part by circumventing the normal control of PP2A by methylation, the Akt and p70/p85 S6K pathways activated by LCMT-1 knockdown are known to be activated by SV40- and polyomavirus-induced transformation [25–27,61–66]. PyMT, PyST, and SVST have all been reported to induce Akt phosphorylation on activating residues [25–27,64–66], whereas PyMT and PyST have been shown to activate the p70 S6K pathway [63,66]. Similar to our present findings, rpS6 phosphorylation induced by these viruses is likely mediated through both Akt-dependent and Akt-independent pathways. For example, PyMT was reported to cause p70 S6K activation and rpS6 phosphorylation through activation of PI3-kinase (and thus Akt) but also to directly induce rpS6 phosphorylation independent of PI3-kinase and p70 S6K [63]. More recent data indicate that PyST can also enhance rpS6 phosphorylation [66].
The pathways impacted by LCMT-1 knockdown in this study are highly relevant to human cancer. Akt activation is common in cancer and, in many cases, correlates with a poor prognosis and resistance to current therapies [67]. As a direct target and important effector of mTOR in the control of cell growth and proliferation, p70 S6K has also been implicated in tumorigenesis [46,68]. Increases in the amount and/or activation of p70 S6K have been reported in multiple cancers or cancer cell lines [69–73]. Therefore, the growth and survival pathways upregulated on loss of LCMT-1 are important not only for SV40- and polyomavirus-mediated transformation but also for human cancer. Our study also demonstrates that modulation of PP2Ac methylation is sufficiently potent to regulate these growth and survival pathways and that LCMT-1 is important for the tumor-suppressive function of PP2A. On the basis of these findings, it seems likely that down-regulation of LCMT-1 amount or activity will be found to contribute to the development of human cancers and that compounds that increase PP2A methylation (e.g., PME-1 inhibitors) will exhibit anticancer activity.
Acknowledgments
The authors thank Kyung Park for excellent technical assistance, Dr. William Hahn and The RNAi Consortium at the Broad Institute for lentiviral shRNA vectors, and Juyeon Hwang, Anita Corbett, and Richard D. Cummings for critical reading of the manuscript. The authors also thank Jocelyn Lee for making the LCMT-1-rescue cDNA.
Abbreviations
- Akt-AA
dominant interfering Akt mutant (T308A/S473A)
- L3
LCMT-1 shRNA
- LCMT-1
leucine carboxyl methyltransferase 1
- PME-1
protein phosphatase methylesterase 1
- PP2A
protein phosphatase 2A
- PP2Ac
PP2A C subunit
- PyST
polyomavirus small tumor antigen
- PyMT
polyomavirus middle tumor antigen
- rpS6
ribosomal protein S6
- S6K
S6 kinase
- SVST
simian virus 40 small tumor antigen
- shRNA
small hairpin RNA
- VC
vector control
Footnotes
This work was supported by a grant from the National Institutes of Health/National Cancer Institute to D.C.P. (CA57327). Dr Pallas is entitled to royalty from the sale of products related to the research described in this article by Millipore, Inc, Santa Cruz Biotechnologies, Inc, Invitrogen Corp, and Novus Biologicals, Inc. In addition, this same author serves as a consultant to Millipore. The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
References
- 1.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haesen D, Sents W, Ivanova E, Lambrecht C, Janssens V. Cellular inhibitors of protein phosphatase PP2A in cancer. Biomed Res. 2012;23:SI 197–SI 211. [Google Scholar]
- 3.Westermarck J, Hahn WC. Multiple pathways regulated by the tumor suppressor PP2A in transformation. Trends Mol Med. 2008;14:152–160. doi: 10.1016/j.molmed.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Westphal RS, Coffee RL, Jr, Marotta A, Pelech SL, Wadzinski BE. Identification of kinase-phosphatase signaling modules composed of p70 S6 kinase-protein phosphatase 2A (PP2A) and p21-activated kinase-PP2A. J Biol Chem. 1999;274:687–692. doi: 10.1074/jbc.274.2.687. [DOI] [PubMed] [Google Scholar]
- 5.Tolstykh T, Lee J, Vafai S, Stock JB. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 2000;19:5682–5691. doi: 10.1093/emboj/19.21.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Tolstykh T, Lee J, Boyd K, Stock JB, Broach JR. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo. EMBO J. 2000;19:5672–5681. doi: 10.1093/emboj/19.21.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei H, Ashby DG, Moreno CS, Ogris E, Yeong FM, Corbett AH, Pallas DC. Carboxymethylation of the PP2A catalytic subunit in Saccharomyces cerevisiae is required for efficient interaction with the B-type subunits Cdc55p and Rts1p. J Biol Chem. 2001;276:1570–1577. doi: 10.1074/jbc.M008694200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu XX, Du X, Moreno CS, Green RE, Ogris E, Feng Q, Chou L, McQuoid MJ, Pallas DC. Methylation of the protein phosphatase 2A catalytic subunit is essential for association of Bα regulatory subunit but not SG2NA, striatin, or polyomavirus middle tumor antigen. Mol Biol Cell. 2001;12:185–199. doi: 10.1091/mbc.12.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Baere I, Derua R, Janssens V, Van Hoof C, Waelkens E, Merlevede W, Goris J. Purification of porcine brain protein phosphatase 2A leucine carboxyl methyltransferase and cloning of the human homologue. Biochemistry. 1999;38:16539–16547. doi: 10.1021/bi991646a. [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Chen Y, Tolstykh T, Stock J. A specific protein carboxyl methylesterase that demethylates phosphoprotein phosphatase 2A in bovine brain. Proc Natl Acad Sci USA. 1996;93:6043–6047. doi: 10.1073/pnas.93.12.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogris E, Du X, Nelson KC, Mak EK, Yu XX, Lane WS, Pallas DC. A protein phosphatase methylesterase (PME-1) is one of several novel proteins stably associating with two inactive mutants of protein phosphatase 2A. J Biol Chem. 1999;274:14382–14391. doi: 10.1074/jbc.274.20.14382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Longin S, Zwaenepoel K, Louis JV, Dilworth S, Goris J, Janssens V. Selection of protein phosphatase 2A regulatory subunits is mediated by the C terminus of the catalytic subunit. J Biol Chem. 2007;282:26971–26980. doi: 10.1074/jbc.M704059200. [DOI] [PubMed] [Google Scholar]
- 13.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55α regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem. 2008;283:1882–1892. doi: 10.1074/jbc.M709585200. [DOI] [PubMed] [Google Scholar]
- 14.Sablina AA, Hector M, Colpaert N, Hahn WC. Identification of PP2A complexes and pathways involved in cell transformation. Cancer Res. 2010;70:10474–10484. doi: 10.1158/0008-5472.CAN-10-2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocher G, Letourneux C, Lenormand P, Porteu F. Inhibition of B56-containing protein phosphatase 2As by the early response gene IEX-1 leads to control of Akt activity. J Biol Chem. 2007;282:5468–5477. doi: 10.1074/jbc.M609712200. [DOI] [PubMed] [Google Scholar]
- 16.Petritsch C, Beug H, Balmain A, Oft M. TGF-β inhibits p70 S6 kinase via protein phosphatase 2A to induce G1 arrest. Genes Dev. 2000;14:3093–3101. doi: 10.1101/gad.854200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallas DC, Shahrik LK, Martin BL, Jaspers S, Miller TB, Brautigan DL, Roberts TM. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell. 1990;60:167–176. doi: 10.1016/0092-8674(90)90726-u. [DOI] [PubMed] [Google Scholar]
- 18.Ogris E, Gibson DM, Pallas DC. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene. 1997;15:911–917. doi: 10.1038/sj.onc.1201259. [DOI] [PubMed] [Google Scholar]
- 19.Yang SI, Lickteig RL, Estes R, Rundell K, Walter G, Mumby MC. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol Cell Biol. 1991;11:1988–1995. doi: 10.1128/mcb.11.4.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheidtmann KH, Mumby MC, Rundell K, Walter G. Dephosphorylation of simian virus 40 large-T antigen and p53 protein by protein phosphatase 2A: inhibition by small-t antigen. Mol Cell Biol. 1991;11:1996–2003. doi: 10.1128/mcb.11.4.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the MAP kinase pathway and induces cell proliferation. Cell. 1993;75:887–897. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 22.Ulug ET, Cartwright AJ, Courtneidge SA. Characterization of the interaction of polyomavirus middle T antigen with type 2A protein phosphatase. J Virol. 1992;66:1458–1467. doi: 10.1128/jvi.66.3.1458-1467.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen W, Possemato R, Campbell KT, Plattner CA, Pallas DC, Hahn WC. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell. 2004;5:127–136. doi: 10.1016/s1535-6108(04)00026-1. [DOI] [PubMed] [Google Scholar]
- 24.Pallas DC, Weller W, Jaspers S, Miller TB, Lane WS, Roberts TM. The third subunit of protein phosphatase 2A (PP2A), a 55-kilodalton protein which is apparently substituted for by T antigens in complexes with the 36- and 63-kilodalton PP2A subunits, bears little resemblance to T antigens. J Virol. 1992;66:886–893. doi: 10.1128/jvi.66.2.886-893.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillet R, Cavard C, Grimber G, Briand P, Joulin V. Hepatic expression of SV40 small-T antigen blocks the in vivo CD95-mediated apoptosis. Biochem Biophys Res Comm. 2001;284:369–376. doi: 10.1006/bbrc.2001.4988. [DOI] [PubMed] [Google Scholar]
- 26.Zhao JJ, Gjoerup OV, Subramanian RR, Cheng Y, Chen W, Roberts TM, Hahn WC. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–495. doi: 10.1016/s1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 27.Yuan H, Veldman T, Rundell K, Schlegel R. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J Virol. 2002;76:10685–10691. doi: 10.1128/JVI.76.21.10685-10691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sontag E, Sontag JM, Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase C ζ signaling targeted by SV40 small t to promote cell growth and NF-κB activation. EMBO J. 1997;16:5662–5671. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno CS, Ramachandran S, Ashby DG, Laycock N, Plattner CA, Chen W, Hahn WC, Pallas DC. Signaling and transcriptional changes critical for transformation of human cells by simian virus 40 small tumor antigen or protein phosphatase 2A B56γ knockdown. Cancer Res. 2004;64:6978–6988. doi: 10.1158/0008-5472.CAN-04-1150. [DOI] [PubMed] [Google Scholar]
- 30.Nunbhakdi-Craig V, Schuechner S, Sontag JM, Montgomery L, Pallas DC, Juno C, Mudrak I, Ogris E, Sontag E. Expression of protein phosphatase 2A mutants and silencing of the regulatory Bα subunit induce a selective loss of acetylated and detyrosinated microtubules. J Neurochem. 2007;101:959–971. doi: 10.1111/j.1471-4159.2007.04503.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee JA, Pallas DC. Leucine carboxyl methyltransferase-1 is necessary for normal progression through mitosis in mammalian cells. J Biol Chem. 2007;282:30974–30984. doi: 10.1074/jbc.M704861200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kitamura T, Ogawa W, Sakaue H, Hino Y, Kuroda S, Takata M, Matsumoto M, Maeda T, Konishi H, Kikkawa U, et al. Requirement for activation of the serine-threonine kinase Akt (protein kinase B) in insulin stimulation of protein synthesis but not of glucose transport. Mol Cell Biol. 1998;18:3708–3717. doi: 10.1128/mcb.18.7.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pallas DC, Schley C, Mahoney M, Harlow E, Schaffhausen BS, Roberts TM. Polyomavirus small t antigen: overproduction in bacteria, purification, and utilization for monoclonal and polyclonal antibody production. J Virol. 1986;60:1075–1084. doi: 10.1128/jvi.60.3.1075-1084.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gordon J, Hwang J, Carrier KJ, Jones CA, Kern QL, Moreno CS, Karas RH, Pallas DC. Protein phosphatase 2a (PP2A) binds within the oligomerization domain of striatin and regulates the phosphorylation and activation of the mammalian Ste-Like kinase Mst3. BMC Biochem. 2011;12:e54. doi: 10.1186/1471-2091-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–468. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 36.Cifone M, Fidler IJ. Correlation of patterns of anchorage-independent growth with in vivo behavior of cells for a murine fibroblast. Proc Natl Acad Sci USA. 1980;77:1039–1043. doi: 10.1073/pnas.77.2.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanevich V, Jiang L, Satyshur KA, Li Y, Jeffrey PD, Li Z, Menden P, Semmelhack MF, Xing Y. The structural basis for tight control of PP2A methylation and function by LCMT-1. Mol Cell. 2011;41:331–342. doi: 10.1016/j.molcel.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hahn WC, Dessain SK, Brooks MW, King JE, Elenbaas B, Sabatini DM, DeCaprio JA, Weinberg RA. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22:2111–2123. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman VH, Shin S. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974;3:355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- 40.Vachon PH. Integrin signaling, cell survival, and anoikis: distinctions, differences, and differentiation. J Signal Transduct. 2011;2011:738137. doi: 10.1155/2011/738137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Peterson RT, Schreiber SL. Translation control: connecting mitogens and the ribosome. Curr Biol. 1998;8:R248–R250. doi: 10.1016/s0960-9822(98)70152-6. [DOI] [PubMed] [Google Scholar]
- 44.Lantier L, Mounier R, Leclerc J, Pende M, Foretz M, Viollet B. Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. FASEB J. 2010;24:3555–3561. doi: 10.1096/fj.10-155994. [DOI] [PubMed] [Google Scholar]
- 45.Hay N, Sonenberg N. Upstream and downstream of mTOR [review] Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 46.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 47.Li SP, Brignole C, Marcellus R, Thirlwell S, Binda O, McQuoid MJ, Ashby D, Chan H, Zhang Z, Miron MJ, et al. The adenovirus E4orf4 protein induces G2/M arrest and cell death by blocking protein phosphatase 2A activity regulated by the B55 subunit. J Virol. 2009;83:8340–8352. doi: 10.1128/JVI.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sontag JM, Sontag E. Regulation of cell adhesion by PP2A and SV40 small tumor antigen: an important link to cell transformation. Cell Mol Life Sci. 2006;63:2979–2991. doi: 10.1007/s00018-006-6300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nho RS, Peterson M. Eukaryotic translation initiation factor 4E binding protein 1 (4EBP-1) function is suppressed by src and protein phosphatase 2A (PP2A) on extracellular matrix. J Biol Chem. 2011;286:31953–31965. doi: 10.1074/jbc.M111.222299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nho RS, Kahm J. β1-Integrin-collagen interaction suppresses FoxO3a by the coordination of Akt and PP2A. J Biol Chem. 2010;285:14195–14209. doi: 10.1074/jbc.M109.052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiely PA, Baillie GS, Lynch MJ, Houslay MD, O'Conner R. Tyrosine 302 in RACK1 is essential for insulin-like growth factor-I-mediated competitive binding of PP2A and B1 integrin and for tumor cell proliferation and migration. J Biol Chem. 2008;283:22952–22961. doi: 10.1074/jbc.M800802200. [DOI] [PubMed] [Google Scholar]
- 52.Xia H, Nho R, Kleidon J, Kahm J, Henke CA. Polymerized collagen inhibits fibroblast proliferation via a mechanism involving the formation of a B1 integrin-protein phosphatase 2A-tuberous sclerosis complex 2 complex that suppresses S6K1 activity. J Biol Chem. 2008;283:20350–20360. doi: 10.1074/jbc.M707489200. [DOI] [PubMed] [Google Scholar]
- 53.De Toni-Costes F, Despeaux M, Bertrand J, Bourogaa E, Ysebaert L, Payrastre B, Racaud-Sultan C. A new α5β1 integrin-dependent survival pathway through GSK3B activation in leukemic cells. PLoS One. 2010;5:e9807. doi: 10.1371/journal.pone.0009807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gendron S, Couture J, Aoudjit F. Integrin α2β1 inhibits Fas-mediated apoptosis in T lymphocytes by protein phosphatase 2A-dependent activation of the MAPK/ERK pathway. J Biol Chem. 2003;278:48633–48643. doi: 10.1074/jbc.M305169200. [DOI] [PubMed] [Google Scholar]
- 55.Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–1264. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 56.Ramel D, Lagarrigue F, Dupruis-Coronas S, Chicanne G, Leslie N, Gaits-Iacovoni F, Payrastre B, Tronchere H. PtdIns5P protects Akt from dephosphorylation through PP2A inhibition. Biochem Biophys Res Commun. 2009;387:127–131. doi: 10.1016/j.bbrc.2009.06.139. [DOI] [PubMed] [Google Scholar]
- 57.Bielinski VA, Mumby MC. Functional analysis of the PP2A subfamily of protein phosphatases in regulating drosophila S6 kinase. Exp Cell Res. 2007;313:3117–3126. doi: 10.1016/j.yexcr.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hombauer H, Weismann D, Mudrak I, Stanzel C, Fellner T, Lackner DH, Ogris E. Generation of active protein phosphatase 2A is coupled to holoenzyme assembly. PLoS Biol. 2007;5:e155. doi: 10.1371/journal.pbio.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortega-Gutierrez S, Leung D, Ficarro S, Peters EC, Cravatt BF. Targeted disruption of the pme-1 gene causes loss of demethylated PP2A and perinatal lethality in mice. PLoS One. 2008;3:e2486. doi: 10.1371/journal.pone.0002486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Puustinen P, Junttila MR, Vanhatupa S, Sablina AA, Hector ME, Teittinen K, Raheem O, Ketola K, Lin S, Kast J, et al. Pme-1 protects extracellular signal-regulated kinase pathway activity from protein phosphatase 2A-mediated inactivation in human malignant glioma. Cancer Res. 2009;69:2870–2877. doi: 10.1158/0008-5472.CAN-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kennedy IM, Leader DP. Increased phosphorylation of ribosomal protein S6 in hamster fibroblasts transformed by polyoma virus and simian virus 40. Biochem J. 1981;198:235–237. doi: 10.1042/bj1980235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blenis J, Erikson RL. Phosphorylation of the ribosomal protein S6 is elevated in cells transformed by a variety of tumor viruses. J Virol. 1984;50:966–969. doi: 10.1128/jvi.50.3.966-969.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talmage DA, Blenis J, Benjamin TL. Polyomavirus middle T antigen induces ribosomal protein S6 phosphorylation through pp60c-src-dependent and -independent pathways. Mol Cell Biol. 1988;8:2309–2315. doi: 10.1128/mcb.8.6.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meili R, Cron P, Hemmings BA, Ballmer-Hofer K. Protein kinase B/Akt is activated by polyomavirus middle-T antigen via a phophatidylinositol 3-kinase-dependent mechanism. Oncogene. 1998;16:903–907. doi: 10.1038/sj.onc.1201605. [DOI] [PubMed] [Google Scholar]
- 65.Dahl J, Jurczak A, Cheng LA, Baker DC, Benjamin TL. Evidence of a role for phosphatidylinositol 3-kinase activation in the blocking of apoptosis by polyomavirus middle T antigen. J Virol. 1998;72:3221–3226. doi: 10.1128/jvi.72.4.3221-3226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Andrabi S, Gjoerup OV, Kean JA, Roberts TM, Schaffhausen B. Protein phosphatase 2A regulates life and death decisions via Akt in a context-dependent manner. Proc Natl Acad Sci USA. 2007;104:19011–19016. doi: 10.1073/pnas.0706696104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Calvo E, Bolos V, Grande E. Multiple roles and therapeutic implications of Akt signaling and cancer. Onco Targets Ther. 2009;2:135–150. doi: 10.2147/ott.s4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jastrzebski K, Hannan KM, Tchhoubrieva EB, Hannan RD, Pearson RB. Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors. 2007;25:209–226. doi: 10.1080/08977190701779101. [DOI] [PubMed] [Google Scholar]
- 69.Nozawa H, Watanabe T, Nagawa H. Phosphorylation of ribosomal p70 S6 kinase and rapamycin sensitivity in human colorectal cancer. Cancer Lett. 2007;251:105–113. doi: 10.1016/j.canlet.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Y, Pan Y, Zhang S, Shi X, Ning T, Ke Y. Increased phosphorylation of p70 S6 kinase is associated with HPV16 infection in cervical cancer and esophageal cancer. Br J Cancer. 2007;97:218–222. doi: 10.1038/sj.bjc.6603838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Castellvi J, Garcia A, Rojo F, Ruiz-Marcellan C, Gil A, Baselga J, Ramon y Cajal S. Phosphorylated 4E binding protein 1: a hallmark of cell signaling that correlates with survival in ovarian cancer. Cancer. 2006;107:1801–1811. doi: 10.1002/cncr.22195. [DOI] [PubMed] [Google Scholar]
- 72.Kwon HK, Bae GU, Yoon JW, Kim YK, Lee HY, Lee HW, Han JW. Constitutive activation of p70 S6K in cancer cells. Arch Pharm Res. 2002;25:685–690. doi: 10.1007/BF02976945. [DOI] [PubMed] [Google Scholar]
- 73.Barlund M, Forozan F, Kononen J, Bubendorf L, Chen Y, Bittner ML, Torhorst J, Haas P, Bucher C, Guido S, et al. Detecting activation of ribosomal protein S6 kinase by complementary DNA and tissue microarray analysis. J NCI. 2000;92:1252–1259. doi: 10.1093/jnci/92.15.1252. [DOI] [PubMed] [Google Scholar]