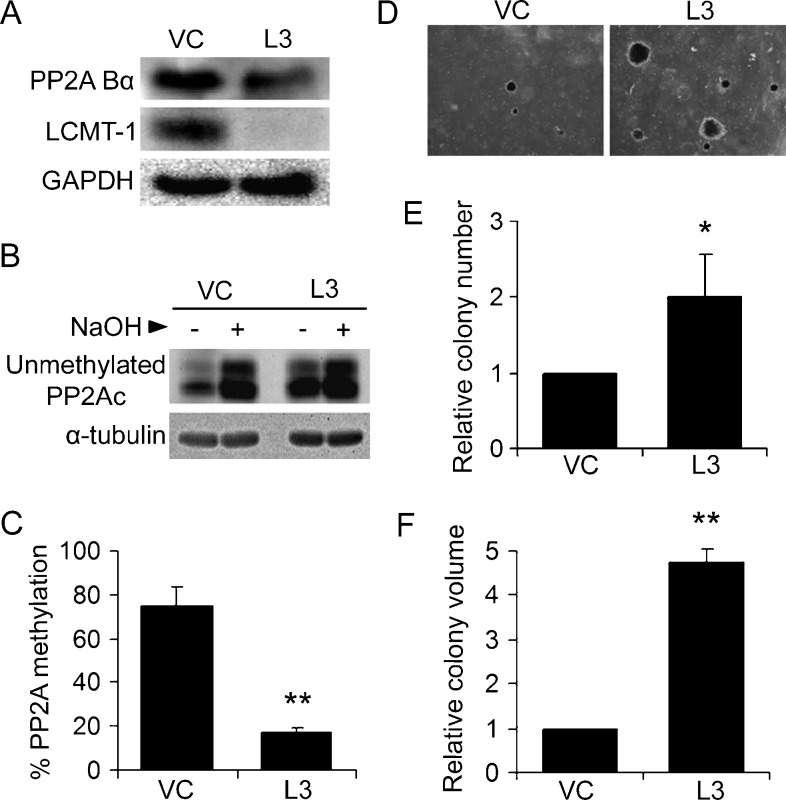
Figure 2.
Knocking down LCMT-1 promotes transformation. (A) Knockdown of LCMT-1 in HEKTERASB56γ cells. HEKTERASB56γ cells stably expressing empty pLKO.1 vector control (VC) or LCMT-1 shRNA (L3) were lysed and LCMT-1 and Bα levels were detected by Western blot analysis. GAPDH was used as loading control. (B and C) PP2Ac is highly unmethylated in the LCMT-1 knockdown line. (B) As described in Materials and Methods, equal volumes of lysates from VC and L3 cells were either treated with preneutralized base solution (- lanes; show unmethylated PP2Ac levels in cells) or base treated to completely demethylate PP2Ac and then neutralized (+ lanes; 100% demethylated controls) before being analyzed by Western blot analysis for the level of unmethylated PP2Ac and α-tubulin (loading control). (C) The percent unmethylated PP2Ac was determined by quantitatively comparing the unmethylated PP2Ac signals in the - and + lanes. Percent methylated PP2Ac was calculated by subtracting percent unmethylated PP2Ac from 100. Graph depicts the average percent methylation of PP2Ac in VC and L3 lines. Error bars represent SDs of three independent experiments. (D) Anchorage-independent growth of VC and L3 cells in soft agar. Photographs show small, single, representative fields within the agar wells. Average colony numbers (E) and average colony volumes (F) were determined as described in Materials and Methods, and data are shown in graphs as fold change relative to VC. Error bars represent SD of three independent experiments performed in triplicate. *P ≤ .05. **P ≤ .01.