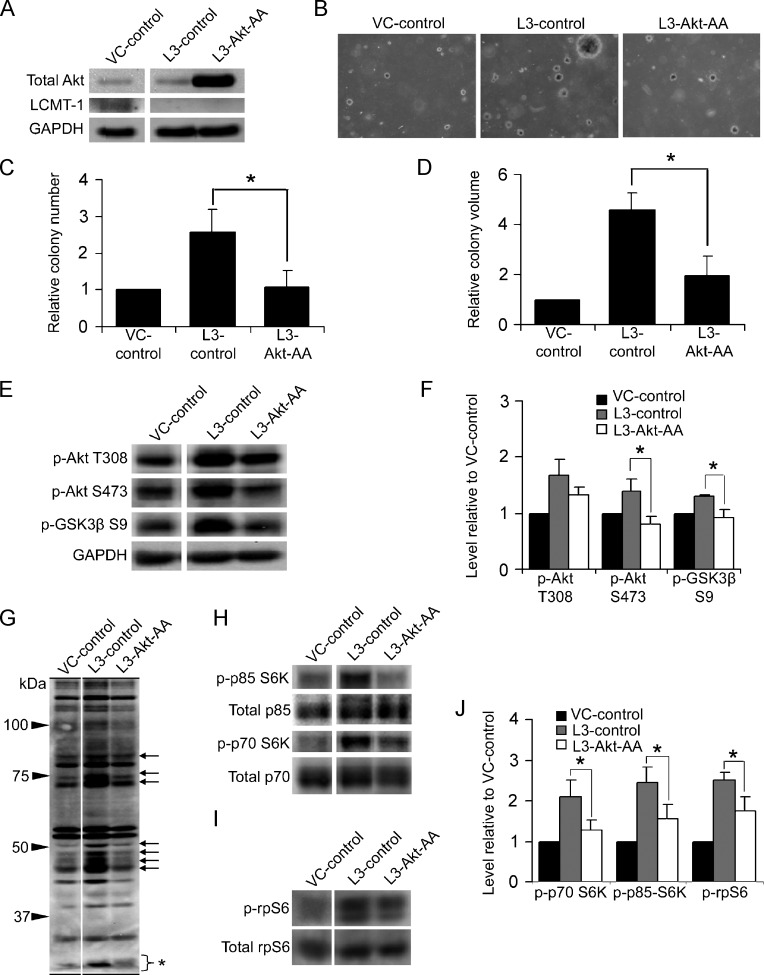
Figure 7.
Akt activation is necessary for the enhanced transformation caused by LCMT-1 knockdown. (A) Lysates from VC and L3 cells stably expressing an empty control plasmid (VC-control and L3-control, respectively) and lysates from L3 cells stably expressing dominant-negative Akt-AA (L3-Akt-AA) were analyzed by Western blot analysis for the expression of Akt-AA, LCMT-1, and GAPDH. (B) knockdownknockdownAnchorage-independent growth of VC-control, L3-control, and L3-Akt-AA cells in soft agar. Photographs show small, single, representative fields within the agars. Average colony numbers (C) and average colony volumes (D) were determined, and data are shown in graphs as fold change relative to VC-control. Error bars represent SD of three independent experiments performed in triplicate. (E) Lysates from VC-control, L3-control, and L3-Akt-AA suspension colonies were analyzed by Western blot analysis for changes in activation of endogenous Akt. GAPDH was used as a loading control. (F) Graph depicts the average fold change in the levels of phospho-Akt T308, phospho-Akt S473, and phospho-GSK3β S9 in three immunoblot experiments relative to VC-control. (G) Probing of VC-control, L3-control, and L3-Akt-AA lysates with phospho-Akt substrate antibody shows that dominant-negative Akt expression prevents the increased phosphorylation of many proteins caused by LCMT-1 knockdown (arrows show examples). The bracket with asterisk indicates phospho-rpS6, which ran at the bottom of this 7.5% SDS-PAGE gel. (H and I) Lysates were probed for changes in p70 and p85 S6K activation and rpS6 phosphorylation. Total p70 and p85 S6K and total rpS6 were used as controls. (J) Graph depicts the average fold change in the levels of phospho-p85, phospho-p70, and phospho-rpS6 in three immunoblot experiments relative to VC-control. Error bars represent SD of three independent experiments. For all graphs: *P ≤ .05. **P ≤ .01.