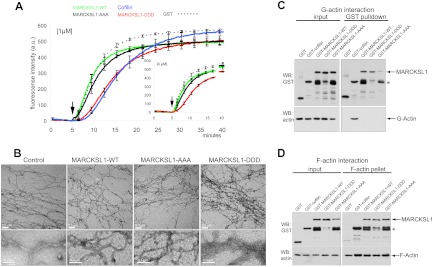
Fig 2.
Pseudophosphorylated MARCKSL1S120D,T148D,T183D induces F-actin bundling. (A) To test whether MARCKSL1 regulated actin filament formation in vitro, we measured the polymerization rate of pyrene-labeled G-actin in the presence of 1 μM or 4 μM (inset) bacterially purified MARCKSL1s or cofilin as a control. After initiation of polymerization (arrows), fluorescence was measured at 20-s intervals. Like cofilin, MARCKSL1-DDD delayed actin polymerization onset. Mean values ± standard errors of the means (SEM) of the results of 3 to 4 repeat experiments are shown. (B) Electron microscope images of negatively stained actin (10 μM) polymerized in the presence of 1 μM GST (control), GST-MARCKSL1, GST-MARCKSL1-AAA, or GST-MARCKSL1-DDD. Only GST-MARCKSL1-DDD induced F-actin bundling. (C) To assess whether MARCKSL1 or MARCKSL1 phosphorylation mutants bind to G-actin, GST-tagged MARCKSL1s or cofilin was incubated in buffer that was nonpermissive with respect to polymerization. GST-MARCKSL1 proteins were isolated and analyzed by Western blotting (WB). GST-MARCKSL1s did not interact with G-actin, but GST-cofilin did. (D) To test whether MARCKSL1 or MARCKSL1 phosphorylation mutants bound F-actin, MARCKSL1s were incubated in actin polymerization-permissive buffer. F-actin was isolated by ultracentrifugation. MARCKSL1-WT, MARCKSL1-DDD, MARCKSL1-AAA, and cofilin each copelleted with F-actin.