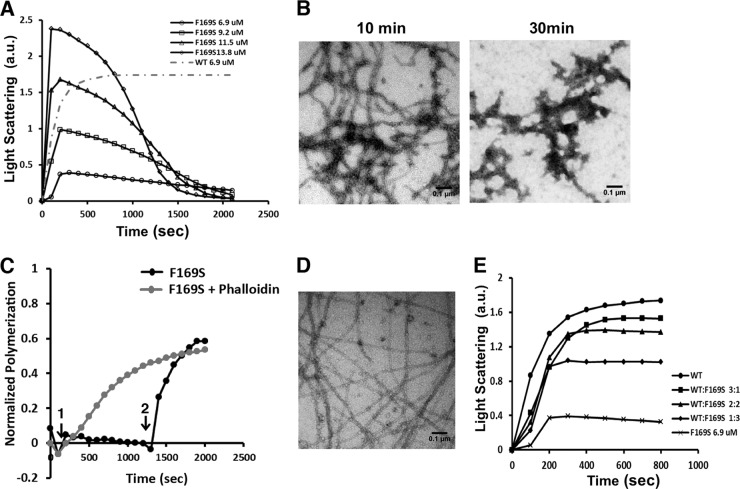
Fig 7.
Yeast F169S mutant actin polymerization analysis reveals destabilized filaments and rescue using phalloidin treatment. (A) F169S actin polymerization and visualization of filaments. F169S actin at the concentration indicated was induced to polymerize by the addition of salt as described in Materials and Methods. The increase in light scattering was recorded with time. The gray dotted line shows the extent of polymerization of a concentration of WT actin equal to the lowest concentration of mutant actin examined. a.u., arbitrary units. (B) A 3-μl aliquot was removed from the 13.8 μM F169S actin polymerization reaction mixture in panel A at the time indicated and visualized by electron microscopy as described in Materials and Methods. Each experiment was repeated at least twice with different actin preparations with essentially identical results. (C) Phalloidin rescues F169S actin filament formation. A 5 μM F169S actin sample was induced to polymerize at time point 1, as in panel A. At time point 2, following filament disassembly, 5 μM phalloidin was added. The gray line indicates polymerization of the mutant actin when phalloidin is added at the beginning of the polymerization assay, which can rescue the polymerization defect. The change in light scattering was recorded with time and normalized to the net change in light scattering caused by the polymerization of 5 μM WT actin. (D) Following attainment of the polymerization steady state, a 3-μl mutant actin sample was removed and visualized by electron microscopy as described in Materials and Methods. (E) Copolymerization of F169S actin with WT actin at different WT/mutant ratios. Solutions of 6.9 mM actin at different F169S/WT actin ratios were induced to polymerize by the addition of salt as described in Materials and Methods. The increase of light scattering was recorded with time. The experiments were repeated twice with similar results.