Fig 2.
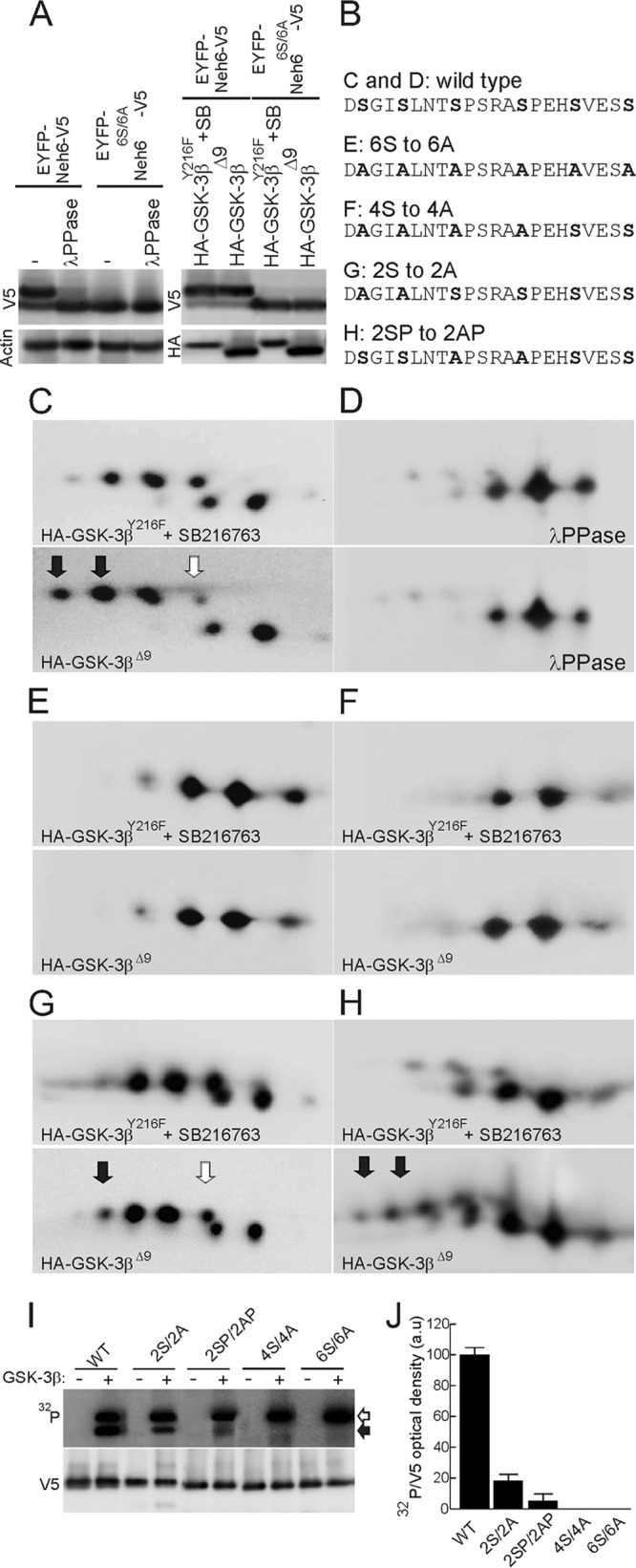
The Neh6 domain is phosphorylated by GSK-3β. (A) (Left) HEK293T cells were transfected with expression vectors for EYFP-mNrf2(317–372)-V5 and mutant EYFP-mNrf2(317–372)6S/6A-V5. Cell lysates were incubated with λPPase as indicated and resolved by one-dimensional SDS-PAGE. (Top) Immunoblot with anti-V5 antibody; (bottom) immunoblot with anti-β-actin antibody to check equal protein loading. (Right) HEK293T cells were cotransfected with expression vectors for EYFP-mNrf2(317–372)-V5 and mutant EYFP-mNrf2(317–372)6S/6A-V5 together with either HA-GSK-3βY216F (plus incubation with SB216763, 10 μM, 3 h), as a negative control, or constitutively active HA-GSK-3βΔ9. (Top) Immunoblot with anti-V5 antibody; (bottom) immunoblot with anti-HA antibody. (B) Comparison of mNrf2 sequences from wild-type and mutant EYFP chimeras examined in panels C to H. (C) 2D-PAGE analysis of EYFP-mNrf2(317–372)-V5 in the presence of either HA-GSK-3βY216F (plus incubation with SB216763) or active HA-GSK-3βΔ9. (D) Same lysates as in C after treatment with λPPase. (E to H) 2D gel analysis of mutant EYFP-mNrf2(317–372)6S/6A-V5 (E), mutant EYFP-mNrf2(317–372)4S/4A-V5 (F), mutant EYFP-mNrf2(317–372)2SP/2AP-V5 (G), and mutant EYFP-mNrf2(317–372)2S/2A-V5 (H), in the presence of HA-GSK-3βY216F (plus incubation with SB216763) or active HA-GSK-3βΔ9. Filled arrows indicate acidic spots that result from GSK-3 phosphorylation; empty arrow indicates spots whose intensity is reduced in the presence of HA-GSK3βΔ9 as a result of protein migration toward that acidic spot. (I and J) In vitro GSK-3β kinase assays on Nrf2-derived substrates. HEK293T cells were transfected with wild-type and mutant EYFP-Neh6-V5, as indicated. Cell lysates were immunoprecipitated with anti-V5 antibodies and then subjected to phosphorylation with recombinant GSK-3β. (Top) 32P autoradiography. Filled and empty arrowheads indicate phosphorylated EYFP-Neh6-V5 and autophosphorylated GSK-3β, respectively. (Bottom) Immunoblots with anti-V5 antibodies showing similar amounts of immunoprecipitated EYFP-Neh6-V5 proteins per reaction. To exclude the possibility that a contaminating kinase in the reaction mixture might be responsible for modifying Nrf2, we performed parallel in vitro kinase assays without recombinant GSK-3β. (J) Densitometric quantification of 32P autoradiography normalized to EYFP-Neh6-V5 densities. Values are means and SEM from three independent reactions per EYFP-Neh6-V5 mutant.
