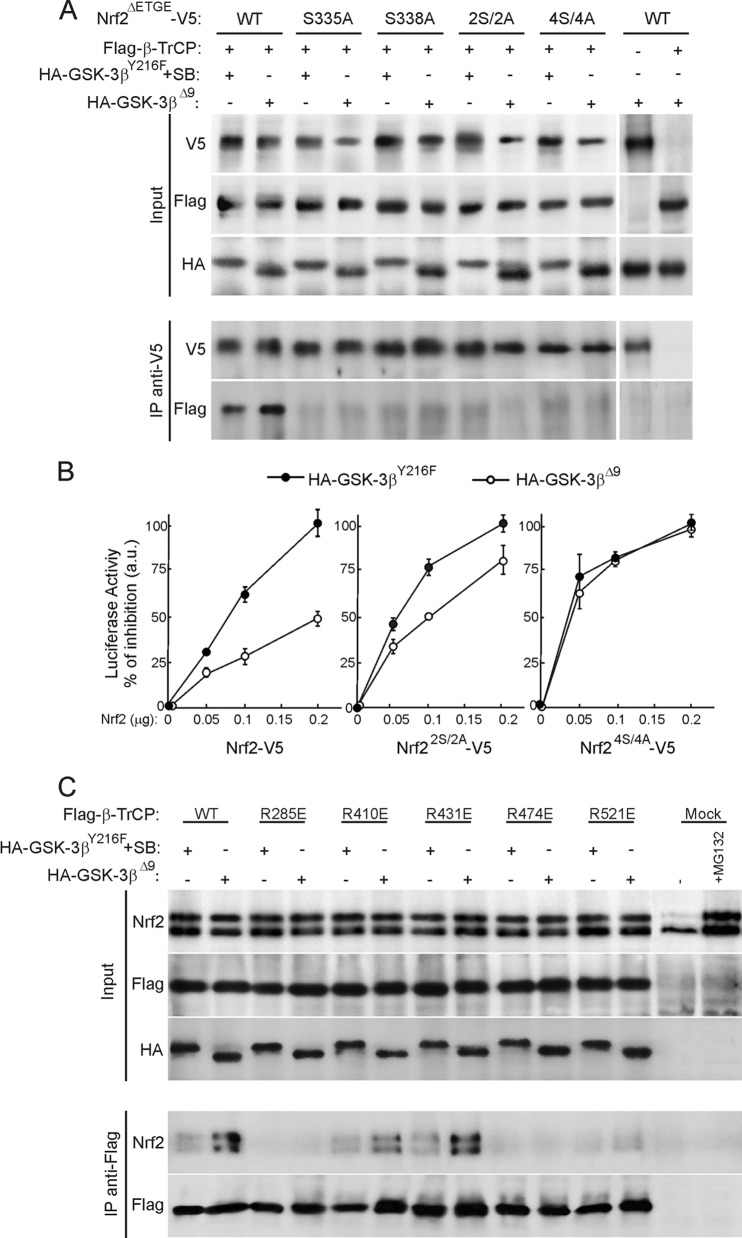
Fig 5.
Identification of critical residues for Nrf2 docking onto β-TrCP. (A) HEK293T cells were cotransfected with Flag-tagged β-TrCP, HA-tagged GSK-3βY216F (plus incubation with SB216763), and GSK-3βΔ9, as indicated, plus V5-tagged mouse Nrf2ΔETGE with either a wild-type Neh6 domain (wt) or mutations of Ser335 to Ala (S335A), Ser338 to Ala (S338A), Ser335 and Ser338 to Ala (2S/2A), or Ser335, Ser338, Ser342, and Ser347 to Ala (4S/4A). Cells were maintained in low serum for 16 h and treated with 40 μM MG132 for the last 3 h before pulldown of protein with anti-V5 antibodies. Lysates from only V5- or HA-transfected HEK293T were incubated with anti-V5 antibodies and G protein to control unspecific binding. (B) Nrf2-null MEFs were cotransfected with ARE-Luc, pTK-Renilla as control vector, HA-tagged GSK-3βY216F, or GSK-3βΔ9, as indicated, and increasing amounts of Nrf2-V5, Nrf22S/2A-V5, or Nrf24S/4A-V5. After 48 h, luciferase activity was determined. Values are means ± SEM from three independent samples. (C) Association of endogenous Nrf2 with Flag-tagged wild-type and mutant β-TrCPs. HEK293T cells were transfected with the Flag-β-TrCP constructs and HA-tagged GSK-3βY216F (plus incubation with SB216763) or GSK-3βΔ9. After 16 h in low-serum medium, cells were treated for 3 h with 40 μM MG132 and then immunoprecipitated with anti-Flag antibody. Samples from cells transfected with empty vector (mock) were included to control unspecific binding.