Fig 1.
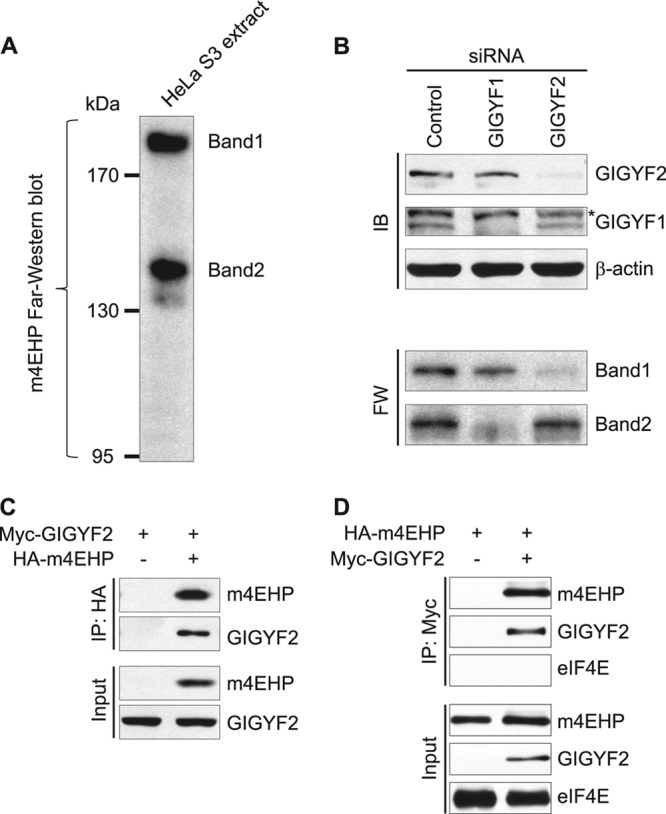
Identification of GIGYF proteins as novel m4EHP-interacting partners. (A) Identification of 4EHP-binding proteins by far-Western analysis. Extracts from HeLa S3 cells were resolved by SDS-PAGE and transferred to nitrocellulose membrane, which was incubated with 32P-labeled recombinant 4EHP. Immunoprecipitates were separated by SDS-PAGE, the gel was stained with Coomassie brilliant blue, and the bands of interest were excised for MS analysis. MS identified GIGYF2 and GIGYF1 as candidates for bands 1 and 2, respectively. (B) GIGYF1 and GIGYF2 were depleted by siRNA in HeLa S3 cells. The levels of both proteins were specifically reduced, as demonstrated by immunoblotting (IB; upper panels). β-Actin served as a loading control. The asterisk denotes a nonspecific band. These lysates were subjected to far-Western analysis (FW; lower panels). (C) HeLa S3 cells were transfected with a Myc-GIGYF2 plasmid, with or without an HA-4EHP plasmid. Interactions were examined by coimmunoprecipitation (Co-IP) with anti-HA antibody, followed by immunoblotting (IB) with anti-Myc and anti-HA antibodies. (D) HeLa S3 cells were transfected with an HA-4EHP plasmid, with or without a Myc-GIGYF2 plasmid. Interactions were examined by Co-IP with anti-Myc antibody, followed by IB with anti-Myc, anti-HA, and anti-eIF4E antibodies. For panels C and D, inputs represent 10% of the total lysate used in the IP assay.
