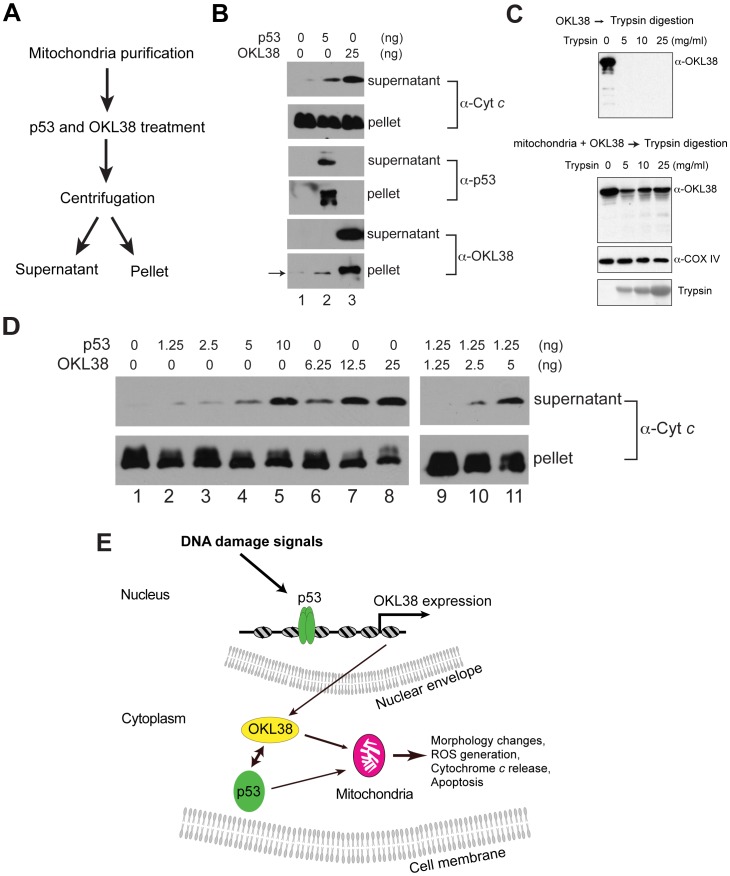
Figure 6. OKL38 and p53 induced cytochrome c release from purified mitochondria in vitro.
(A) Biochemical scheme used to analyze the effect of p53 and OKL38 on cytochrome c release from mitochondria. (B) Effects of p53 and OKL38 on cytochrome c release (top 2 panels), and the detection of p53 (middle 2 panels) and OKL38 (bottom 2 panels) in both soluble and pellet fractions by Western blot. Arrow denotes low levels of endogenous OKL38 in mouse liver mitochondria. (C) Western blot analyses of OKL38 digestion by trypsin without (upper panel) or with prior incubation with mitochondria (Lower panel). COX IV protein was monitored to show that mitochondrial protein is protected from trypsin digestion. The amount of trypsin in the reaction was detected by Ponceau S staining. (D) Western blot analyses of the amount of cytochrome c in the supernatant and pellet fractions after mitochondria were incubated with p53 and/or OKL38 at various concentrations. (E) A model of the function of OKL38 and its cooperation with p53 during DNA damage to regulate mitochondria-mediated cell death. Our data favor a model that after DNA damage, p53 activates the expression of OKL38. On the other hand, OKL38 protein can interact with p53 in cytoplasm and target each other to mitochondria to regulate mitochondrial morphology, function, and cell death.