Figure 3. Affinity capture and western blotting of Topors.
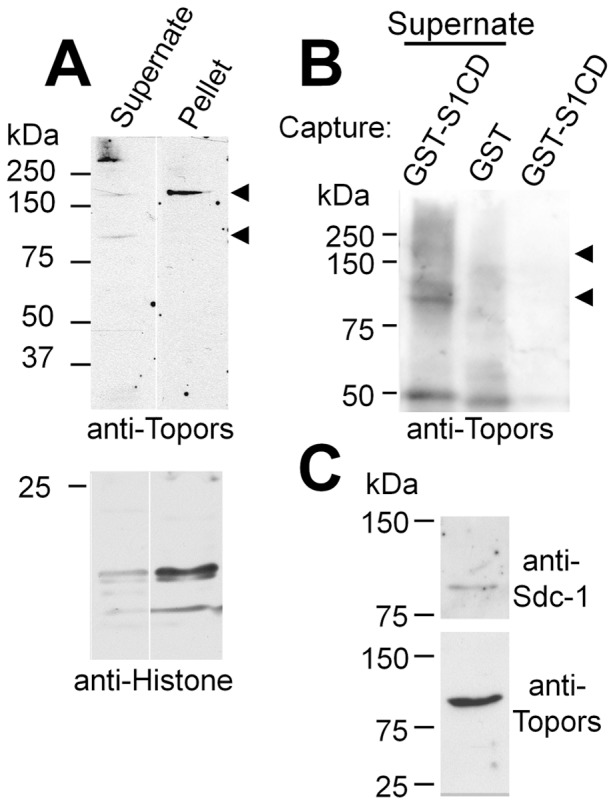
(A) Western blot of NMuMG cell detergent lysate (supernate) and detergent-insoluble material (pellet) probed with chicken anti-Topors antibody. Two major bands (∼110 and ∼175 kDa (arrowheads)) are apparent. The larger band is prevalent in detergent-insoluble fractions, which contain relatively more histone proteins (lower blot), consistent with enhanced presence of nuclear proteins. (B) Capture of endogenous Topors from cell lysates by GST-S1CD. GST-S1CD or GST alone were adsorbed to glutathione-agarose beads and incubated with detergent lysates of 3T3 cells. Anti-Topors-reactive bands captured by GST-S1CD were detected in lysates of 3T3 cells that overexpress Topors (first lane, arrows), and were similar in size to the major bands seen in western blots of whole cell lysates. Similar bands were not captured by GST alone (lane 2), nor are they present in the GST-S1CD reagent (lane 3). Non-specific bands are seen at ∼55 kDa. (C) Co-precipitation of Topors with polyhistidine-tagged Sdc-1 isolated by metal-ion affinity chromatography. Metal ion-affinity gel bound material was isolated from detergent extracts of cells that express polyhistidine-tagged Sdc-1. Parallel samples were run on SDS-PAGE, either directly or after heparinase and chondroitinase digestion and transferred to nitrocellulose. Parallel blots were probed with chicken anti-Topors or, for digested samples, with anti Sdc-1.
