Abstract
Objective
To determine the relevance of S100A12 expression to human thoracic aortic aneurysms (TAA) and type A dissection (TAAD) and to study mechanisms of S100A12 mediated dysfunction of aortic smooth muscle cells.
Background
Transgenic expression of pro-inflammatory S100A12 protein in murine aortic smooth muscle causes thoracic aneurysm in genetically modified mice.
Methods
Immunohistochemistry of aortic tissue (n=50) for S100A12, myeloperoxidase and caspase 3 was examined, and S100A12 mediated pathways were studied in cultured primary aortic smooth muscle cells.
Results
We found S100A12 protein expressed in all cases of acute TAAD, and in approximately 25% of clinically stable TAA cases. S100A12 tissue expression was associated with increased length of stay in patients undergoing elective surgical repair for TAA despite similar preoperative risk as determined by EuroSCORE. Reduction of S100A12 expression in human aortic smooth muscle cells using shRNA attenuates gene and protein expression of many inflammatory and apoptosis regulating factors. Moreover, genetic ablation of the receptor for S100A12, RAGE, in murine aortic smooth muscle cells abolished cytokine- augmented activation of caspase 3 and smooth muscle cell apoptosis in S100A12-expressing cells.
Conclusion
S100A12 is enriched in human thoracic aortic aneurysms and dissections. Reduction of S100A12 or genetic ablation of its cell surface receptor RAGE in aortic smooth muscle resulted in decreased activation of caspase 3 and in reduced apoptosis. By establishing a link between S100A12 expression and apoptosis of aortic smooth muscle cells, this study identifies novel S100A12 signaling pathways and indicates that S100A12 may be a useful molecular marker and possible target for treatment for human aortic diseases.
Keywords: Thoracic aortic aneurysm and dissection, S100 proteins, RAGE, aortic smooth muscle cells, cell death
Introduction
Thoracic aortic aneurysms (TAA) encompass a broad range of degenerative, genetic, structural, and acquired disease states and can be complicated by potentially life threatening type A dissection (TAAD). There is growing evidence of genetic variation in genes known to be critically important in the development of TAA including FBN1 encoding fibrillin 1, TGFBR1/2 encoding TGFβ receptors, ACTA2 and MHY11 that encode smooth muscle α- actin and myosin heavy chain, respectively, that predisposes to aortic diseases (1). We recently reported that transgenic mice engineered to express human S100A12 in the aortic smooth muscle develop thoracic aneurysms (2), similar to mouse models of Marfan syndrome (3). S100A12 is a “pro-inflammatory” protein that activates the Receptor for Advanced Glycation Endproducts (RAGE, (4)) and accelerates atherosclerosis in apolipoprotein A deficient mice (5). Moreover, S100A12 gene expression in human peripheral blood cells is one of the most predictive genes for obstructive coronary artery disease (6) and S100A12 is expressed in smooth muscle and inflammatory cells in ruptured coronary artery plaque of victims of sudden cardiac death (7), suggesting a critical role of S100A12 in vascular disease and atherothrombosis. While the expansion of the aortic wall occurs slowly and is often clinically asymptomatic, dissection of the medial layer with bleeding within and along the aortic wall occurs suddenly and leads to major complications including rupture, ischemia and arterial thrombosis with embolization, accounting for at least 16,000 deaths annually in the United States (1,8). The precipitating mechanisms underlying dissection are incompletely understood and include hemodynamic factors, endothelial factors, as well as dysfunctional smooth muscle cells. Moreover, inflammation and the presence of inflammatory cells within the aortic media have been recently emphasized as potential mediator for dissections (8,9). Since S100A12 is a potent pro-inflammatory and chemoattractant protein, and S100A12 transgenic mice develop TAA, we wished to examine S100A12 expression in human aortic diseases. We found strong expression of S100A12 in inflammatory cells and in smooth muscle cells in all cases of thoracic aneurysms with type A dissection, and in approximately 25% in clinically stable TAA. Furthermore, studies on cultured human aortic smooth muscle cells (HASMC) obtained from patients with TAA/TAAD show a direct role of S100A12 in mediating apoptosis. Together these data demonstrate that S100A12 is up regulated in TAAD and may contribute to the pathogenesis of TAAD by initiating apoptosis of smooth muscle cells, at least in part via increased oxidative stress.
Methods
The University of Chicago Medical Center Pathology tissue bank was searched between 2007 and 2010 and 20 cases of elective TAA repair were randomly chosen. To validate our findings from the initial 20 randomly chosen patients, 30 additional patients with TAA/TAAD were studied with either emergent surgery for type A aortic dissections or elective surgery for large aneurysms (>5.5cm). All patients had no preoperative diagnosis of known coronary artery diseases or peripheral artery disease. Retrospective data was collected by reviewing patient medical records, and operative risk prior to surgery was calculated using the additive and the logistic EuroSCORE (European System for Cardiac Operative Risk Evaluation, http://www.euroscore.org). Normal control aortic tissue was from obtained from heart donors (n=3, 2 male, age 44±12), aortic valve replacement for stenotic tricuspid aortic valve with normal aorta (n=2, 2 male, age 48±8) and elective left ventricular assist device (n=1, female, age 32). Serial histological sections were prepared from paraffin embedded aortic tissue blocks and stained for hematoxylin-eosin, Verhoeff Van Giessen and Movat, and immunochemistry stained with αS100A12 IgG (abcam 37657), α-myeloperoxidase IgG (Abcam 9535) and α- smooth muscle actin (Sigma). Staining for S100A12 was semiquantified by two blinded investigators (DD, MAHB) as absent (0), mild (1+), moderate (2+) or intense (3+) in cells that either was positive or negative for myeloperoxidase staining. Loss of aortic medial elastic fibers were graded as 1 (trace), 2 (mild), 3 (moderate) and 4 (full thickness loss) as previously reported by Dr. W.C.Roberts (10,11).
Aortic Smooth muscle cells
Human aortic smooth muscle cells (HASMC) were cultured from operatively excised aortic tissue (bicuspid aortic valve n=3, TGFBR1 mutation n=2, FBN1 mutation n=2, unknown etiology n=3) as previously described (2). Control HASMC were obtained from heart donors (n=3), aortic valve replacement for stenotic tricuspid aortic valve with normal aorta (n=2) and elective left ventricular assist device (n=1). Murine aortic smooth muscle cells (MASMC) were obtained from hemizygous S100A12 transgenic mice of the C57BL6/J strain previously generated (2), and from mice mated with C57BL6/J mice lacking RAGE (Rage-/-), supplied by Dr. Ann Marie Schmidt (New York University, NY). Only cell cultures testing negative for contaminating leukocytes (α-CD45.2 antibody from Pharmingen #559985; and α-CD68 Pharmingen #556059) were propagated. SMC characterized by staining for smooth muscle actin (Sigma) from passage 4-7 were used for experiments. If indicated, soluble RAGE, the extra cellular ligand binding domain of RAGE (20ug/ml (R&D), bovine serum albumin (BSA, 20 ug/ml, Sigma), α lipoic acid (1, 10μM and 100 μM, AstraZeneka), or Diphenyleneiodonium (DPI, 1 and 10 μM, Sigma) was added prior to stimulation with LPS (100 ng/ml, Sigma), TNF-α (10 ng/ml, Pierce), or αFas IgG (CH 11, Millipore). Where indicated, HASMC were transfected with shRNA-S100A12 and control shRNA (SA Bioscience) using lipofectamine method (Clontech). Transfected HASMC were selected three days later by FACS-sorting employing the GFP-tag co-expressed in the shRNA plasmids prior to further analysis.
Immunoblotting was performed on whole cell lysate (Pierce) for S100A12 (Abcam 37657), b-actin, caspase 3, Fas (Cell Signaling), FAS L (Cell Signaling) and DFFA (Millipore) and semi-quantitative analysis was performed using ImageQuant TL software (Amersham). Quantitative RT-PCR was performed from high quality total RNA (Qiagen) after transcription to cDNA (Qiagen whole transcriptome assay) using SYBR GreenER (Invitrogen) amplification with an IQ5 cycler (BioRAD). Pathway focused micro array RT-PCR was purchased from SA Bioscience. Primers were designed on the primer bank MGH library. Relative levels of gene expression were calculated: Relative mRNA expression= 2exp (ΔCT target gene- ΔCT housekeeping gene). Immunofluorescent assays for Caspase 3 (BioVision) was analyzed on a Fluostar Optima plate reader (BMG Labtech). For immunofluorescence microscopy, SMC were grown on glass cover slips, fixed with 20% ice cold methanol and analyzed using primary antibody anti 8-oxo-dG IgG (Trevigen) or used for the TUNEL assay (Roche). Cells were imaged by confocal laser microscopy (Zeiss 500 Meta) and fluorescent intensity was analyzed in five high power fields, at least in 200 cells.
The study and all procedures were carried out with the approval of the Chicago Institutional Review Board and the Institutional Animal Care and Use Committee.
Statistical analysis
Descriptive statistics (percentage, mean, and standard deviation), crude comparison based on x2 statistics, and plots were used to describe the patient cohort. All continuous data are reported as mean ± SD and discrete variables were summarized by percentage. All experiments were performed in at least 3 replicates per group and all cell culture experiments were repeated three times. Independent sample t-test and one-way analysis of variance were used for mean comparison between two or multiple groups, respectively. The Spearman rank correlation, two sided Chi-square test for two independent proportions and Fisher exact test were also performed to describe association between different outcome variables. We applied the Bonferroni correction to the 0.05 significance level to adjust for multiple comparisons.
Results
S100A12 protein is significantly up-regulated in human thoracic aortic type A dissection
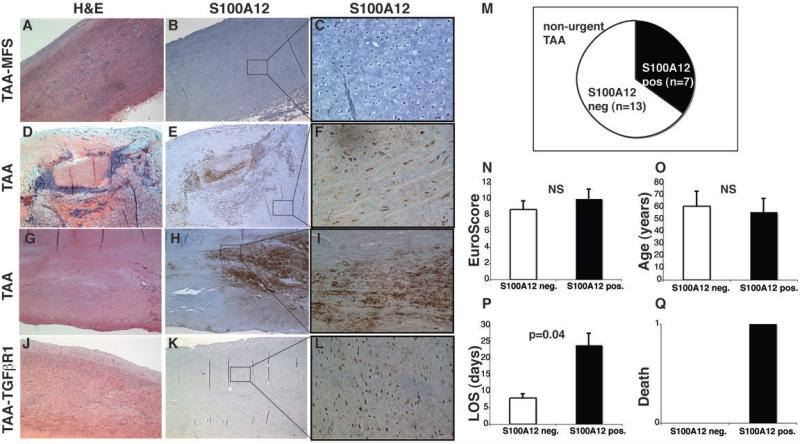
We first studied S100A12 expression in aortic tissue collected from 20 patients undergoing elective repair for TAA (Table 1). All patients had a computed tomography of the chest or an angiogram with a TAA of at least 4.5 cm and were referred for non-urgent surgery. Of those 20 patients, 7 had a bicuspid aortic valve, two had Marfan syndrome, one had familial TAA and 10 patients had no specific diagnosis prior to surgery. We found S100A12-positive cells within the medial layer in 7 cases, and representative images are shown in Figure 1. S100A12 expression was observed in necrotizing aortitis (Figure 1E), atherosclerosis (Figure 1H) and in cases with histological evidence of sub acute dissection (Figure 1K), while we did not observe S100A12 expression in the medial layer in the other 13 cases of TAA (Figure 1B) or in non-aneurysmal control aortic tissue (n=5, not shown). Importantly, all patients in this group underwent elective and non-urgent repair of TAA. Notably, the seven patients with S100A12 expression in the aorta had a significant increased length of hospitalization post surgery with a mean duration of 24 days (range 9-49) compared to a mean duration of 8 days (range 5-13) in the thirteen S100A12-negative TAA patients (p=0.04). The postoperative complications we encountered included prolonged ventilation due to respiratory failure (n=2) and multiorgan failure with need for dialysis (n=1). One patient died on postoperative day 15 due to multiorgan failure occurring after revision for aortic graft dehiscence in the S100A12 positive group compared to no deaths in the S100A12 negative group. To account for preoperative co-morbidities, we calculated the European System for Cardiac Operative Risk Evaluation (EuroSCORE), a widely used score to predict mortality from cardiac surgery(12). We found no difference in the EuroSCORE between the S100A12 positive and the S100A12 negative cases (10.0 and 8.7, respectively, p=0.62, Figure 1N). Moreover, there was also no difference in age between the two groups. This suggests that expression of S100A12 in aortic tissue in TAA is a marker of postoperative complications with increased length of stay.
Table 1.
Patient's characteristics. S100A12 was expressed in 7 of 20 elective TAA cases randomly chosen and was found in the aortic medial layer, localized to areas of chronic/subacute dissection, aortitis or atherosclerosis. All patients with acute TAAD stained positive for S100A12 in the medial layer, and 4 of 16 large and elective TAA showed S100A12 stain in the medial layer.
| Non urgent TAA (n=20) | Urgent TAAD (n=14) | Non urgent TAA > 5.5 cm (n=16) | |
|---|---|---|---|
| 1. cohort (n=20) | 2. cohort (n=30) | ||
| Male, n (%) | 11(55) | 5 (35) | 9 (56) |
| Age (range) | 57 (19-82) | 55 (34-74) | 54 (22-65) |
| Euro-Score | 9.3 (4-16) | 17.3 (9-21)* | 9.1 (5-15) |
| WBC | 8.1 | 12.4* | 6.8 |
| Creatine | 1.2 | 1.3 | 1.2 |
| S100A12 pos Histology | 7 cases: dissection (n=4) necrotizing aortitis (n=2) atherosclerosis (n=1) | 14 cases*: dissection (n=14) | 4 cases: dissection (n=2) medial degeneration (n=2) |
| S100A12 neg Histology | 13 cases: medial degeneration (n=12) dissection (n=1) | 0 cases | 12 cases: medial degeneration (n=10) dissection (n=2) |
p=0.0001
Figure 1. S100A12 expression in stable thoracic aortic aneurysms (TAA).
(A) Histopatholgy with hematoxylin & eosin (H&E) and S100A12 immunostaining of four representative patients, showing no S100A12 in C and S100A12 (brown color) in the medial layer in F, I, L. scale bar = 10μm. Quantification of S100A12 expression in tissue (M), calculated logistic Euroscore (N), age (O), length of stay (LOS, P), and death (Q) of 20 patients undergoing elective, non-urgent surgery for thoracic aortic aneurysms.
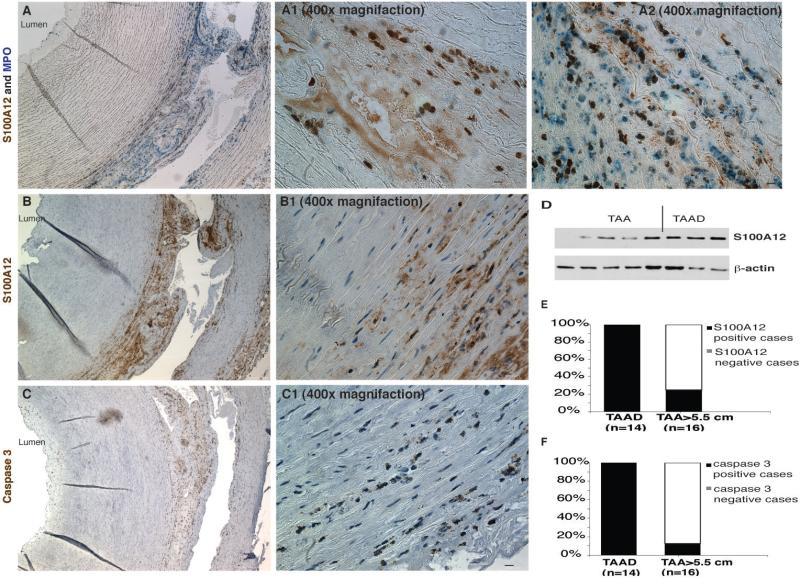
In the S100A12-positive cases we found S100A12 mostly expressed in the medial aortic layer associated with inflammatory changes or histological evidence of subacute dissection (Figure 1D-L). In contrast, in TAA cases showing medial degeneration but without histological evidence of dissection, S100A12 was not or only minimally expressed; except in one of the 13 S100A12 negative-TAA case we found histological evidence of chronic dissection. Therefore, to directly test the hypothesis that S100A12 is a marker of aortic dissection, we selected 14 cases of preoperatively known TAAD undergoing urgent surgical repair from the histology archive. As expected, these TAAD patients had higher preoperative EuroSCORE (17.3 with range of 9-12, Table 1) and upon histological examination all 14 cases had S100A12-positive cells in the medial layer of the ascending thoracic aorta. Co-staining for myeloperoxidase (MPO), a peroxidase enzyme expressed in all myeloid cells and abundantly enriched in neutrophil granulocytes, revealed S100A12 expression in MPO-positive inflammatory cells and also in MPO-negative smooth muscle cells (Figure 2). To account for factors possibly related to “disease severity”, we selected 16 more cases of advanced TAA with aneurysms exceeding 5.5 cm undergoing elective and non-urgent repair of TAA. We found S100A12 expression in 4 cases (25%) of TAA>5.5 cm compared to 100% in TAAD (p<0.0001). The S100A12 expression in those large TAA's was localized to a tear with dissecting split of the medial layer (n=2), and in two cases to medial degeneration without dissection. The intensity of S100A12 stain was overall less in the clinically stable TAA cases compared to the cases with acute dissection. The specificity of the staining for S100A12 was verified by immunoblot analysis of protein isolated from aortic medial layer showing the expected 12 kDa size (Figure 2D). To further determine a possible association of S100A12 expression in aneurysmal aortic tissue with other key processes of aortic remodeling, we graded the integrity of aortic medial fibers. The morphology of elastic fibers serves as a good marker of chronic aortic remodeling, such as replacement with fibrous tissue and deposits of mucoid material, and are easily discernable and the grading of elastic fiber loss is highly reproducible. This is in contrast to acute type A dissection, where abnormal ascending aortic elastic fibers uncommonly precede acute dissection (10,11). Supplement Figure 1 shows the frequency of elastic fiber degradation in elective TAA with regards to S100A12 protein expression. For smaller aneurysms, intact or only minimal fiber loss (grade 0-1) are more prevalent among S100A12 negative aneurysms (61% vs. 0%, p=0.007), while advanced elastolysis grade 3-4 is more prevalent among S100A12 positive TAA (57% vs 8%, p=0.015). This relationship was not observed among larger TAAs. There was no difference in high-grade elastolysis in large TAA>5.5 cm with and without S100A12 expression (50% and 33%, respectively, p=0.55). This data suggests that S100A12 expression in aneurismal aorta is associated with proteolysis of elastic fibers since all S100A12 positive TAAs had evidence of grade 2 or higher elastic fiber degradation.
Figure 2. S100A12 expression in thoracic aortic aneurysm dissection (TAAD).
(A) Co-staining for S100A12 (brown color) and myeloperoxidase (MPO, blue color) shows S100A12 expression in MPO-negative smooth muscle cells (A1) and in inflammatory MPO-positive cells (A2). (B, B1) Expression of S100A12 is most intense near the dissection tear, and colocalizes to caspase 3 (C and C1) positive cells near the dissection tear. scale bar = 10μm. (D) Verification of S100A12 protein by immunoblot. Quantification of S100A12 (E) and caspase 3 (F) expression in patients with unstable TAAD (n=14) and stable TAA >5.5 cm (n=16).
Moreover, expression of activated caspase 3, an enzyme known to play a central role in the execution-phase of cell apoptosis, was also present in all TAAD tissue and co-localized with S100A12 to the area near the dissection tear in the tunica media. Caspase 3 was not detected in S100A12 negative cases (Figure 2).
Furthermore, we examined cultured aortic smooth muscle cells and found increased mRNA and protein expression of cell death receptor Fas, and its ligand FasL in the cell lines that also express S100A12 (3.5 fold increase in Fas protein, and 2.6 fold increase in FasL protein. p<0.01; Supplement Figure 2). As expected, we found enhanced mRNA for several members of the TGF-ß signaling pathways in human aortic smooth muscle cells harvested from TAA compared to control HASMC, however there was no difference with regard to S100A12 expression in the different TAA-HASM cell lines (Supplement Figure 2).
Reduction of endogenous S100A12 in cultured human smooth muscle cells attenuates apoptosis
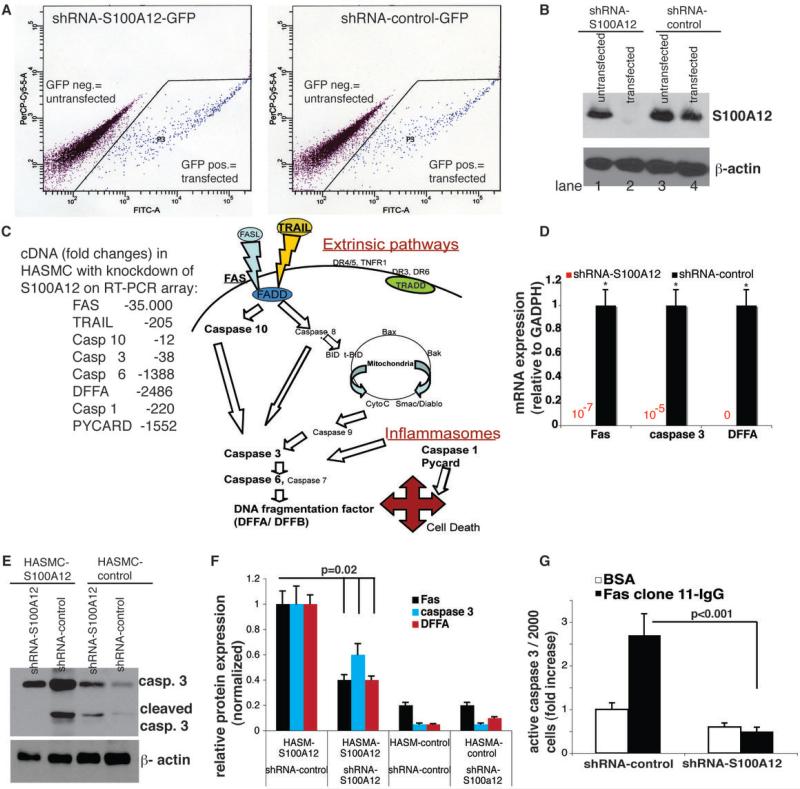
The relationship of S100A12 to markers of apoptosis was studied in cultured human aortic smooth muscle cells (HASMC) harvested from patients undergoing surgical repair for TAA/TAAD and in control HASMC from patients undergoing cardiac surgery without TAA/TAAD. We found variable expression of S100A12 in HASMC from TAA/TAAD by immunoblotting, and no expression of S100A12 in control HASMC (data not shown). Three independent HASMC lines with endogenous S100A12 expression were studied; one carried a known mutation in the FBN1gene (R529X) harvested from a patient with Marfan syndrome, and one cell line carried a mutation in the TGFBR1 (977A>C, K326T) from a patient with familial TAA, and a third HASMC-TAA line was without specific genetic diagnosis. HASMC were transiently transfected with interfering shRNA to knock down endogenous S100A12 or with control shRNA. After isolation of the GFP-positive transfected cells by flow cytometry, we confirmed complete absence of S100A12 in the HASMC transfected with shRNA-S100A12 and endogenous S100A12 expression in HASMC transfected with control shRNA (Figure 3). We next examined gene expression in the FBN1R529X HASMC endogenously expressing S100A12 (=shRNA-control transfected cells) and compared to FBN1R529X HASMC with reduction of S100A12 (=shRNA-S100A12 transfected cells) using pathway focused RT-PCR microarrays for apoptosis and inflammation (SA Bioscience). We identified broad down regulation of apoptosis and inflammation regulating genes. Of the 84 genes present on the microarray plate, 23 genes were >2-fold and 49 genes were <2-fold down regulated on the apoptosis array and 19 genes were >2-fold and 56 genes <2-fold on the inflammation micro array (Supplement Table 1). Since we found strong reduction in Fas, caspase 3 and DNA fragmentation factor A (DFFA), we next examined these genes using quantitative RT-PCR in independent cDNA samples of the three S100A12-expressing HASMC lines. As shown in Figure 3D, reduction of S100A12 abolished mRNA for Fas, caspase 3 and DFFA (p<0.0001). Moreover, reduction of S100A12 suppressed protein expression for Fas, cleaved caspase 3, and DFFA by 40-60% in S100A12 expressing HASMC (p=0.02) but had no effect on S10012-negative HASMC (Figure 3E, F). To confirm a critical role of S100A12 on caspase 3 activation, we stimulated S100A12-expressing HASMC with apoptosis initiating monoclonal Fas IgG and observed a 2.8 fold increase in active caspase 3 using a immunofluorescent active caspase 3 assay. ShRNA reduction of S100A12 reduced Fas-IgG stimulated active caspase 3 by 80% (Figure 3G, p<0.001). These data suggest that S100A12 is a critical nodal point in many apoptosis and inflammation regulating genes.
Figure 3. ShRNA mediated reduction of endogenous S100A12 in HASMC attenuates apoptosis.
(A) Flow cytometry for GFP expression was used to isolate transfected HASMC with shRNA-S100A12 or shRNA-control, (B) shRNA-S100A12 (lane 2) but not shRNA-control (lane 4) abolishes S100A12 protein in HASMC. (C) Changes in gene expression of apoptosis and inflammation regulating genes after reduction of S100A12 in HASMC-Fbn1R529X tested by pathway focused RT-PCR microarray (complete data shown in Supplement Table 1). S100A12 expressing HASMC lines (n=3) were transfected with shRNA and after selection by flow cytometry analyzed for (D) gene expression for Fas, caspase 3 and DNA fragmentation factor A (DFFA), and for protein content of Fas, caspase 3, and DFFA (E, F). (G) Quantification for capase 3 using immunofluorescent activity assay. * p<0.0001.
S100A12 is induced in HASMC by cytokines, and knockdown of S100A12 attenuates cytokine-induced activation of caspase 3 in a partial RAGE dependent manner
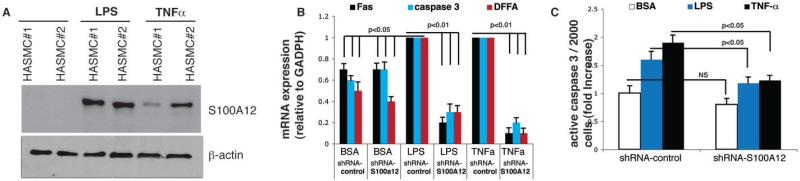
We previously showed that lipopolysaccaride (LPS) induces S100A12 in HASMC in agreement with other studies that showed induction of S100A12 in response to cytokine stimulation (13,14). Moreover, studies of the S100A12 gene indicated C/EBPb and protein kinase C as critical regulators of transcription in response to LPS (15). As shown in Figure 4A, stimulation with low dose LPS or with the distinct cytokine TNFα induced S100A12 in normal HASMC. PCR amplification detected cDNA for Fas, caspase 3 and DFFA in both, control HASMC (BSA) and in cytokine treated HASMC (LPS or TNFα), with significant higher gene expression for Fas, caspase 3 and TNFα in cytokine treated HASMC compared to control HASMC (p<0.05 for each gene). Importantly, co-treatment with shRNA-S100A12 attenuated cytokine induced gene expression for Fas, caspase 3 and DFFA by 70-90% (p<0.01 for each gene) while control shRNA had no effect on cytokine induced up regulation of apoptosis controlling genes (Figure 4B). Quantification of active caspase-3 showed a 1.6 fold increase in LPS treated cells and a 1.9 fold increase in TNFα treated cells compared to baseline; this was attenuated in HASMC co-treated with shRNA-S100A12 by 74% and 65%, respectively (p<0.05, Figure 4C).
Figure 4. Knockdown of S100A12 in HASMC attenuates cytokine induced apoptosis.
(A) S100A12 is induced in control HASMC by lipopolysaccaride (100 ng/ml for 16 hours, lane 3 and 4) and TNF-α (20 ng/ml for 16 hours, lane 5 and 6). (B) Gene expression for Fas, caspase 3 and DFFA measured by qRT-PCR in HASMC treated as indicated. (C) Quantification for capase 3 using immunofluorescent activity assay.
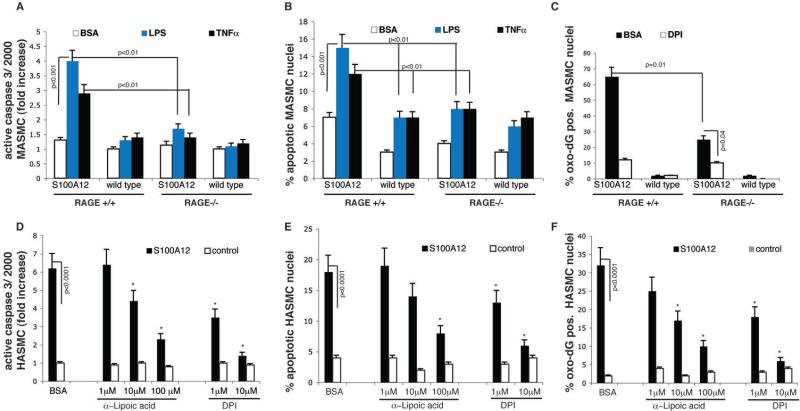
To study potential mechanisms involved in S100A12-mediated apoptosis, we employed murine aortic smooth muscle cells (MASMC). Briefly, transgenic mice with expression of human S100A12 in aortic smooth muscle were previously generated (2) and now crossed with mice lacking RAGE (16). We previously reported that transgenic S100A12-MASMC had higher levels of oxidative stress and IL-6 secretion among other changes characteristic of a phenotypic switch from a contractile to a synthetic phenotype; and these changes were attenuated by co-treatment with soluble RAGE (2), the S100A12 ligand-binding domain of RAGE, thus preventing activation of cell surface RAGE. We now expanded these findings by measuring activated caspase 3 in S100A12 expressing MASMC and found a slight increase compared to wild type MASMC (1.3 fold, p=0.04). Importantly, upon stimulation with low dose LPS (100ng/ml), or low dose TNFα (20ng/ml), the S100A12-MASMC showed strong activation of caspase 3 (4-fold and a 2.9-fold, respectively) compared to a 1.3 and 1.4-fold increase in wild type MASMC (Figure 5A). Moreover, cytokine stimulation caused enhanced DNA fragmentation as visualized by TUNEL staining in S100A12-MASMC but only minimally in WT-MASMC at the studied concentrations for LPS and TNF-α (15% vs 7%, and 12% vs 7%, respectively p<0.01, Figure 5B). Most importantly, murine aortic smooth muscle cells lacking RAGE were protected from the synergistic effects of S100A12 and LPS /TNFα stimulation on caspase 3 activation and cell apoptosis. Activated caspase 3 was reduced in cytokine stimulated S100A12- MASMC by 58% and 52% in cells lacking RAGE compared to S100A12- MASMC with intact RAGE signaling (p<0.01, Figure 5A). This was paralleled with a significant reduction of TUNEL positive MASMC (Figure 5B). Together, we found that SM22α-targeted forced expression of human S100A12 in MASMC markedly enhances cytokine-induced apoptosis, and this was dependent on RAGE, the cell surface receptor for S100/calgranulins endogenously expressed on smooth muscle cells.
Figure 5. S100A12 mediated apoptosis in smooth muscle cells is mediated by RAGE and oxidative stress.
(A-C) MASMC from S100A12 and wild type mice with intact RAGE signaling (RAGE+/+) and deleted RAGE (RAGE-/-) were treated as indicated with control (bovine serum albumine, BSA), lipopolysaccaride (100 ng/ml) or TNF-α (20ng/ml). (D-F) S100A12 expressing HASMC and control HASMC were treated as indicated with antioxidants, α-lipoic acid and DPI. Active caspase 3 was measured using an immunofluorescence assay for active caspase 3 (A, D), TUNEL assay determined % of apoptotic nuclei (B, E), and nuclei with oxidized DNA stained with α-8-oxo-dG IgG (F) and indexed to total number of nuclei per high power field (C,F). (*p<0.01 compared to untreated S100A12 positive HASMC).
Given the importance of reactive oxygen species to inflammation, cell death and apoptosis, we probed oxidative stress related changes. We found an increase in oxidized DNA using immunofluorescence microscopy with anti 8-hydroxy-2-deoxyguanosine IgG with approximately 60% of all nuclei staining positive for this marker of oxidative damage to the DNA in S100A12 transgenic MASMC, compared to a less than 2% baseline in wild type MASMC (p<0.001, Figure 5C). S100A12-MASMC lacking RAGE had significantly reduced number of oxidized nuclei (25%, p=0.01) compared to S100A12-MASMC with intact RAGE signaling, confirming that the pro-oxidative effect of S100A12 is mediated, at least in part, by RAGE. Treatment with diphenylene iodonium (DPI), an inhibitor of NADPH-oxidase also attenuated DNA oxidation, even in cells lacking RAGE (12% and 25%, p=0.04). This data suggests that oxidative stress in cultured smooth muscle cells is a critical downstream effect of S100A12 mediated in large part by RAGE and by NADPH-oxidase. Therefore, we tested the effects of antioxidant compounds on apoptosis markers in human aortic smooth muscle cells with endogenous S100A12 expression (harvested from TAA/TAAD) and in control human aortic smooth muscle cells without S100A12 expression. As shown in Figure 5D-F, pretreatment with α-lipoic acid or DPI reduced active caspase 3, apoptosis index determined by TUNEL-assay and reduced the index for cells with oxidized DNA in a dose dependent manner.
Discussion
An inflammatory component was previously identified in TAA and TAAD, composed primarily of T-cells and macrophages (9). Our study demonstrates that S100A12, a cytokine like protein with pro-inflammatory signaling, is highly enriched in myeloid and in smooth muscle cells in type A aortic dissection. Based on our cell culture data and known pathologic effects of S100A12 on smooth muscle cells, we speculate that S100A12 is highly pathogenic in TAA and may predispose to aortic dissection. S100A12 is also known as calgranulin C or EN-RAGE (extracellular newly identified RAGE binding protein) because it activates pattern recognition receptor RAGE (4) and is likely to also activate toll like receptor 4, as this was recently shown for the homolog S100A8/9 (17). Moreover, S100 proteins are endogenously expressed in myeloid cells and at high levels in neutrophil granulocytes and are implicated in the antibacterial and antifungal defense (18) by activating the p67phox /NADPH-oxidase system to initiate a respiratory burst in phagocytes (19). Similar to its endogenous role in phagocytes, our laboratory recently showed binding of S100A12 to NADPH-oxidase subunit Nox-1 in vascular smooth muscle cells (5), and importantly, Nox-1 inhibition attenuated S100A12 mediated dysfunction of aortic SMC (20).
Our laboratory recently showed a pro-apoptotic function of S100A12 with increased expression of FAS, caspase 10 and caspase 3 in human airway SMC (21) and thereby extended previous studies showing that excess of S100A8/9 induces apoptosis and cell death in a variety of cell types (22,23). Moreover, Boyd et al. found a 40-fold increase in S100A8/9 mRNA in cardiomyocytes in a mouse model of sepsis (24), and S100B was up regulated in ischemic cardiomyocytes (25). Importantly, in both studies up-regulation of S100 proteins was associated with increased cell death and tissue damage that was attenuated by interruption of RAGE signaling. Our studies presented here demonstrate that down regulation of the ligand S100A12 attenuates cell death of SMC with pathologic endogenous expression of S100A12, and in SMC with cytokine-induced expression of S100A12. These findings support the hypothesis that S100A12 is an important regulator of cell survival and cell death.
S100/calgranulins are involved in the regulation of numerous intracellular activities such as protein phosphorylation, enzyme activities, cell proliferation and differentiation, cytoskeletal rearrangement and membrane organization, protection from oxidative stress, and regulation of intracellular Ca+2 homeostasis, and have been implicated in intercellular regulation of chemotaxis, phagocytosis and immunregulation. Therefore, the specificity for S100/calgranulin mediated effects likely depends on the tissue distribution and cell type. This view is supported by recent findings in mice lacking S100A9 where constitutive deletion of murine S100A9 in apolipoprotein E deficient mice attenuated atherosclerosis (26), while the lack of S100A9 in bone marrow derived cells did not attenuate atherosclerosis in LDL-receptor deficient mice (27). Therefore, pathological expression of S100/calgranulins within the vasculature may be an important accelerator of vascular disease, particularly since S100A12 is not present in normal vascular tissue, but is highly up regulated in smooth muscle cells of diseased blood vessels, such as in human ruptured coronary artery plaques (7), in human atherosclerosis (5), and as shown in this study also in type A aortic dissections and to a lesser degree in clinically stable TAA. It is intriguing that TAA/TAAD is such a focal disease, and because there is no access to distal vascular tissue other than the surgical removed aneurysmal tissue in a given patient with TAA/TAAD, we can not exclude the possibility that S100A12 could be expressed in other vascular beds beyond the aneurysmal thoracic aorta in those patients with TAA/TAAD. It is interesting to note that S100A12 expression in explanted SMC persist over several cell culture passages, and this finding deserves further studies. We speculate that epigenetic changes occurring within the S100 gene cluster may control S100 protein expression. This view is supported by the presence of several CpG islands within the S100 gene cluster and within the first intron of the S100A12 gene (28). Therefore methylation and demethylation of regulatory DNA could explain the cell specific expression of S100 proteins, which is frequently up-or downregulated in various pathological states, including cancer and vascular diseases.
S100A12 is more than an inflammatory biomarker. It causes dysfunction of vascular smooth muscle with increase in matrix metalloproteinase-2 protein, interleukin-6, transformig growth factor-β signaling, and reduction of contractile fibers leading to the development of thoracic aortic aneurysms in transgenic mice expressing human S100A12 driven by the smooth muscle 22-α promoter (2), demonstrating a clear pathological effect of S100A12 in vascular smooth muscle. S100 proteins and other ligands to RAGE and toll like receptors, such as HMGB1 protein, have previously been implicated in the process of sterile inflammation associated with dying cells (29). Our report for the first time shows that knockdown of S100A12 attenuates many apoptosis and inflammation regulating genes, including Fas-mediated apoptosis pathways. One interesting finding in our study was the strong attenuation of inflammasome-associated pathways, including PYCARD and caspase 1 after reducing S100A12. Caspase 1 is activated in response to multiple stimuli, but once activated, results in a conserved program of cell death referred as pyroptosis (30). S100 proteins and other damage associated molecular pattern molecules, are good candidate molecules to activate caspase 1 and further exploration of these pathways could possibly shed light on the intracellular function of S100/calgranulins.
The pro-apoptotic function of S100A12 is mediated, at least in part, by its cell surface receptor RAGE, which is expressed in many cells including SMC, endothelial cells, and inflammatory (31). There is mounting evidence of RAGE being a disease amplifier for initiation and progression of vascular diseases (reviewed in (32)), including aortic aneurysms (33). RAGE mediated cell perturbation is at least in part depended on redox-sensitive signals such as the translocation of redox-sensitive nuclear factor kappa B, which for example is attenuated in stimulated endothelial cells by pretreatment with antioxidant alpha lipoic acid (34). Using α lipoic acid and diphenyleneiodonium (DPI) we show marked inhibition of S100A12 mediated cell death. This is in agreement with a recent studies by Kim et al. demonstrating the ability of α lipoic acid to reduce vascular smooth muscle cell apoptosis by restoring intracellular redox status (35). Indeed, oxidative stress plays a key role in the pathogenesis of thoracic aortic dissections as recently demonstrated by several studies. For example, Liao et al. used comparative proteomics and identified 126 proteins differentially expressed in the aortic media of patients with dissections and with normal aortas. Interestingly, increased lipid peroxidation and a more than 50% reduction in superoxide dismutase in aortic type A-dissections was one of the most important changes noticed in this study (36).
The coexistence of inflammatory cells with markers of apoptotic vascular cell death in the media of ascending aortas with aneurysms and type A dissection was previously noted by several investigators (8,9). However, it remains unknown if apoptosis of medial smooth muscle cells occurs prior to dissection as a primary event leading to weakening and rupture of the aortic wall, or if alternatively as suggest by Roberts et al. the intraluminal arterial pressure in the false luminal channel compresses the adjacent media and induces cell death after, not before acute dissection (11).
In summary, critical pathways involved in TAA and progression to TAAD include immunological processes, such as T-cell and natural killer cell pathways, oxidative stress, depletion of vascular smooth muscle through the process of apoptosis, and the destruction of the extracellular matrix by matrix metalloproteinases (1). Here we show evidence that S100A12 is highly expressed in the medial layer in acute type A aortic dissection. Since all TAAD expressed S100A12 regardless of the heterogeneity present in this sample group, we speculate that S100A12 expression in aortic smooth muscle is a common response to multi-factorial injury. This is supported by our findings in “clinically stable” TAA, where we found S100A12 expression in approximately 25%, most commonly associated with histological evidence of dissection. Alternatively, it is possible that S100A12 is a marker of the inflammatory changes that occur in the aortic wall after acute or chronic dissection, rather than being a predisposing factor for dissection. This underscores the complex biology of aortic disease and histological examination of aneurysmal or dissected aortic tissue is inevitable unlikely to determine the temporal relationship between vascular inflammation and aortic dissection. The mechanisms by which S100A12 gets up-regulated in human aortic diseases is not completely understood, but likely is in response to various inflammatory signals since we demonstrated a robust induction of S100A12 in normal human aortic smooth muscle cells upon treatment with LPS and TNFα. Further studies using aortic tissue, rather than in cultured aortic smooth muscle cells from patients with thoracic aneurysms are needed to examine the impact of S100A12 on key vascular proteins, such as fibrillin, collagen and others. One limitation of our study is the small number and the heterogeneity within the control group. Importantly, reduction of S100A12 attenuates many apoptosis and inflammation regulating genes in human aortic smooth muscle cells with endogenous S100A12 expression, and in cytokine-primed murine aortic smooth muscle cells with forced expression of human S100A12. S100A12 mediated cell death is, at least in part, dependent on RAGE and NADPH-oxidase generated oxidative stress, since apoptosis is attenuated in smooth muscle cells lacking RAGE and in human aortic smooth muscle cells treated with antioxidants α lipoic acid and DPI.
Supplementary Material
Acknowledgements
We thank Dr. Ann Marie Schmidt for the RAGE-/- mice. We thank Dr. Sydeaka Watson for statistical advice. The authors are grateful to the patients and their physicians involved in this study.
Funding Source:
This work was supported by funding from the National Institute of Health (MAHB: K08 HL090917-02, EMM: 5R01HL078926-05), the American Heart Association (DD: 11UFEL762052), the National Marfan Foundation, and by the Doris Duke Charitable Foundation (MAHB and EMM). MAHB is a recipient of the Doris Duke Clinical Scientist Development Award.
Non Standard Abbreviations and Acronyms
- EuroSCORE
European System for Cardiac Operative Risk Evaluation
- HASMC
human aortic smooth muscle cells
- LPS
lipopolysaccaride
- MASMC
murine aortic smooth muscle cells
- MFS
Marfan syndrome
- MPO
myeloperoxidase
- RAGE
Receptor for Advanced Glycation End products
- SMC
smooth muscle cells
- TAA
Thoracic aortic aneurysms
- TAAD
Thoracic aortic aneurysm dissection
- TGFβ
transforming growth factor beta
- FasL
Fas ligand
- BMP
Bone morphogenic protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There is no relationship with industry
Reference
- 1.Hiratzka LF, Bakris GL, Beckman JA, et al. ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010;121:e266–369. doi: 10.1161/CIR.0b013e3181d4739e. [DOI] [PubMed] [Google Scholar]
- 2.Hofmann Bowman M, Wilk J, Heydemann A, et al. S100A12 mediates aortic wall remodeling and aortic aneurysm. Circ Res. 2010;106:145–54. doi: 10.1161/CIRCRESAHA.109.209486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–21. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann MA, Drury S, Fu C, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann Bowman MA, Gawdzik J, Bukhari U, et al. S100A12 in vascular smooth muscle accelerates vascular calcification in apolipoprotein E-null mice by activating an osteogenic gene regulatory program. Arterioscler Thromb Vasc Biol. 2011;31:337–44. doi: 10.1161/ATVBAHA.110.217745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg S, Elashoff MR, Beineke P, et al. Multicenter validation of the diagnostic accuracy of a blood-based gene expression test for assessing obstructive coronary artery disease in nondiabetic patients. Ann Intern Med. 2010;153:425–34. doi: 10.7326/0003-4819-153-7-201010050-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burke AP, Kolodgie FD, Zieske A, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–71. doi: 10.1161/01.ATV.0000131783.74034.97. [DOI] [PubMed] [Google Scholar]
- 8.He R, Guo DC, Estrera AL, et al. Characterization of the inflammatory and apoptotic cells in the aortas of patients with ascending thoracic aortic aneurysms and dissections. J Thorac Cardiovasc Surg. 2006;131:671–8. doi: 10.1016/j.jtcvs.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 9.He R, Guo DC, Sun W, et al. Characterization of the inflammatory cells in ascending thoracic aortic aneurysms in patients with Marfan syndrome, familial thoracic aortic aneurysms, and sporadic aneurysms. J Thorac Cardiovasc Surg. 2008;136:922–9. 929, e1. doi: 10.1016/j.jtcvs.2007.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts WC, Vowels TJ, Ko JM, et al. Comparison of the structure of the aortic valve and ascending aorta in adults having aortic valve replacement for aortic stenosis versus for pure aortic regurgitation and resection of the ascending aorta for aneurysm. Circulation. 2011;123:896–903. doi: 10.1161/CIRCULATIONAHA.110.972406. [DOI] [PubMed] [Google Scholar]
- 11.Roberts WC, Vowels TJ, Kitchens BL, et al. Aortic medial elastic fiber loss in acute ascending aortic dissection. Am J Cardiol. 2011;108:1639–44. doi: 10.1016/j.amjcard.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa T, Kosaki A, Kimura T, et al. The regulation of EN-RAGE (S100A12) gene expression in human THP-1 macrophages. Atherosclerosis. 2003;171:211–8. doi: 10.1016/j.atherosclerosis.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 14.Mahajan N, Bahl A, Dhawan V. C-reactive protein (CRP) up-regulates expression of receptor for advanced glycation end products (RAGE) and its inflammatory ligand ENRAGE in THP-1 cells: inhibitory effects of atorvastatin. Int J Cardiol. 2010;142:273–8. doi: 10.1016/j.ijcard.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Chen H, Cheng L, Yang S, et al. Molecular characterization, induced expression, and transcriptional regulation of porcine S100A12 gene. Mol Immunol. 2010;47:1601–7. doi: 10.1016/j.molimm.2010.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Harja E, Bu DX, Hudson BI, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE-/- mice. J Clin Invest. 2008;118:183–94. doi: 10.1172/JCI32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loser K, Vogl T, Voskort M, et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat Med. 16:713–7. doi: 10.1038/nm.2150. [DOI] [PubMed] [Google Scholar]
- 18.Gottsch JD, Eisinger SW, Liu SH, Scott AL. Calgranulin C has filariacidal and filariastatic activity. Infect Immun. 1999;67:6631–6. doi: 10.1128/iai.67.12.6631-6636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berthier S, Paclet MH, Lerouge S, et al. Changing the conformation state of cytochrome b558 initiates NADPH oxidase activation: MRP8/MRP14 regulation. J Biol Chem. 2003;278:25499–508. doi: 10.1074/jbc.M209755200. [DOI] [PubMed] [Google Scholar]
- 20.Gawdzik J, Mathew L, Kim G, Puri TS, Hofmann Bowman MA. Vascular Remodeling and Arterial Calcification Are Directly Mediated by S100A12 (EN-RAGE) in Chronic Kidney Disease. Am J Nephrol. 2011;33:250–259. doi: 10.1159/000324693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofmann Bowman MA, Heydemann A, Gawdzik J, Shilling RA, Camoretti-Mercado B. Transgenic expression of human S100A12 induces structural airway abnormalities and limited lung inflammation in a mouse model of allergic inflammation. Clin Exp Allergy. 2011;41:878–89. doi: 10.1111/j.1365-2222.2011.03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull. 2003;26:753–60. doi: 10.1248/bpb.26.753. [DOI] [PubMed] [Google Scholar]
- 23.Ghavami S, Kerkhoff C, Chazin WJ, et al. S100A8/9 induces cell death via a novel, RAGE-independent pathway that involves selective release of Smac/DIABLO and Omi/HtrA2. Biochim Biophys Acta. 2008;1783:297–311. doi: 10.1016/j.bbamcr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Boyd JH, Kan B, Roberts H, Wang Y, Walley KR. S100A8 and S100A9 mediate endotoxin-induced cardiomyocyte dysfunction via the receptor for advanced glycation end products. Circ Res. 2008;102:1239–46. doi: 10.1161/CIRCRESAHA.107.167544. [DOI] [PubMed] [Google Scholar]
- 25.Tsoporis JN, Izhar S, Leong-Poi H, Desjardins JF, Huttunen HJ, Parker TG. S100B interaction with the receptor for advanced glycation end products (RAGE): a novel receptor-mediated mechanism for myocyte apoptosis postinfarction. Circ Res. 2010;106:93–101. doi: 10.1161/CIRCRESAHA.109.195834. [DOI] [PubMed] [Google Scholar]
- 26.Croce K, Gao H, Wang Y, et al. Myeloid-related protein-8/14 is critical for the biological response to vascular injury. Circulation. 2009;120:427–36. doi: 10.1161/CIRCULATIONAHA.108.814582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Averill MM, Barnhart S, Becker L, et al. S100A9 differentially modifies phenotypic states of neutrophils, macrophages, and dendritic cells: implications for atherosclerosis and adipose tissue inflammation. Circulation. 2011;123:1216–26. doi: 10.1161/CIRCULATIONAHA.110.985523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lesniak W. Epigenetic regulation of S100 protein expression. Clin Epigenetics. 2011;2:77–83. doi: 10.1007/s13148-011-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annu Rev Immunol. 2010;28:321–42. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergsbaken T, Fink SL, Cookson BT. Pyroptosis: host cell death and inflammation. Nat Rev Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw SS, Schmidt AM, Banes AK, Wang X, Stern DM, Marrero MB. S100B-RAGE-mediated augmentation of angiotensin II-induced activation of JAK2 in vascular smooth muscle cells is dependent on PLD2. Diabetes. 2003;52:2381–8. doi: 10.2337/diabetes.52.9.2381. [DOI] [PubMed] [Google Scholar]
- 32.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–53. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang F, Kent KC, Yamanouchi D, et al. Anti-receptor for advanced glycation end products therapies as novel treatment for abdominal aortic aneurysm. Ann Surg. 2009;250:416–23. doi: 10.1097/SLA.0b013e3181b41a18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bierhaus A, Chevion S, Chevion M, et al. Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes. 1997;46:1481–90. doi: 10.2337/diab.46.9.1481. [DOI] [PubMed] [Google Scholar]
- 35.Kim H, Kim HJ, Lee K, et al. Alpha-lipoic acid attenuates vascular calcification via reversal of mitochondrial function and restoration of Gas6/Axl/Akt survival pathway. J Cell Mol Med. 2011 doi: 10.1111/j.1582-4934.2011.01294.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao M, Liu Z, Bao J, et al. A proteomic study of the aortic media in human thoracic aortic dissection: implication for oxidative stress. J Thorac Cardiovasc Surg. 2008;136:65–72. e1–3. doi: 10.1016/j.jtcvs.2007.11.017. 72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.