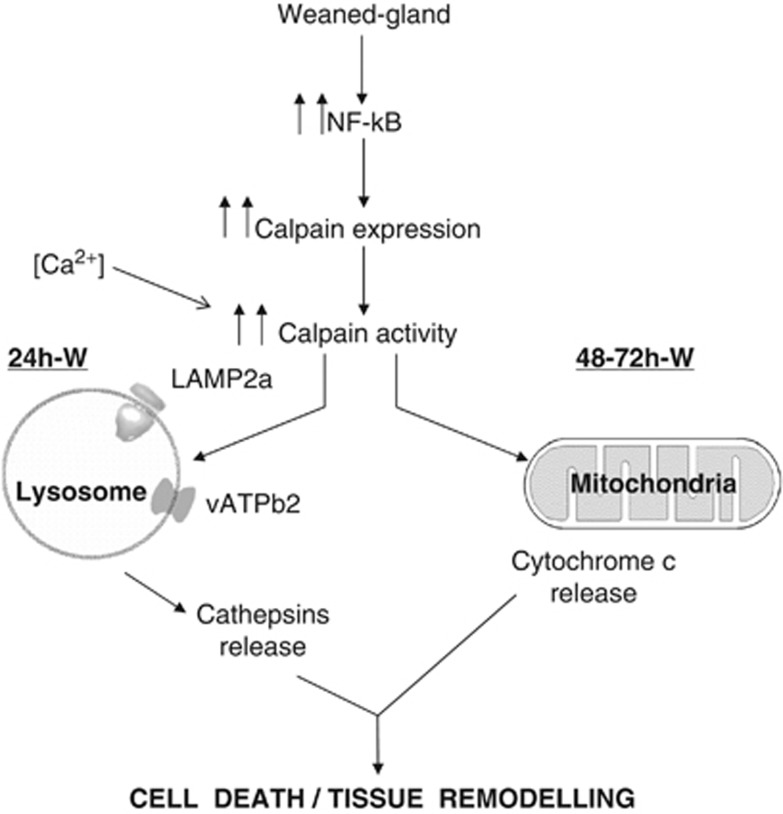
Figure 8.
Hypothetical model of the findings described in this paper. Calpain activation during weaning is involved in both lysosomal permeabilization and mitochondrial destabilization, triggering mammary gland involution. Forced weaning induces NF-κB activation that translocates to the nucleus and promotes expression of downstream target genes such as calpains. Besides, calcium accumulates within the cells and results in the activation of calpains. During the first stage of mammary gland involution, calpain-1 is traslocated to the lysosomal membrane, where it cleaves the cytosolic tail of Lamp2 and other membrane proteins such as VATB2, contributing to destabilizing lysosomes and releasing cathepsin D into the cytosol. This cleavage is prevented by calpeptin and other calpain inhibitors. At the second phase of involution, mitochondrial calpain-1 facilitates mitochondrial permeabilization, thus inducing cytochrome c release and triggering caspase-dependent apoptosis