TABLE 1.
Selectivity of inhibitors of CYP2A enzymes as evaluated by binding affinity and inhibitor dissociation constants
| Inhibitor | P450 | Kd (Binding Mode)a | 2A13 Kd/2A6 Kd | Kid | Mechanism (α Value) | 2A13 Ki/2A6 Ki |
|---|---|---|---|---|---|---|
| μM | μM | |||||
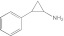 Tranylcypromine Tranylcypromine |
2A13 | 2.3 (II) | 1.2 | 6.5 ± 1.2 | Competitive | 49 |
| 2A6 | 2.0 (II) | 0.13 ± 0.02 | Mixed (α = 10) | |||
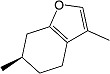 (R)-Menthofuran (R)-Menthofuran |
2A13 | 0.58 (I) | 0.13 | 54 ± 12 | Mixed (α = 4.3) | 27 |
| 2A6 | 4.5 (I) | 2.0 ± 0.44 | Mixed (α = 4.3) | |||
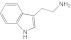 Tryptamine Tryptamine |
2A13 | 5.2 (II) | 2.3 | 16 ± 3.5 | Competitive | 9.4 |
| 2A6 | 2.3 (II) | 1.7 ± 0.12 | Competitive | |||
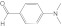 DMABA DMABA |
2A13 | 0.65 (I) | 0.96 | 17 ± 5.0 | Mixed (α = 13) | 4.6 |
| 2A6 | 0.68 (I) | 3.6 ± 0.83 | Mixed (α = 5.5) | |||
 PEITC PEITC |
2A13 | 0.43 (I)b | 0.064 | 3.8 ± 1.6 | Mixed (α = 0.20) | 2.2 |
| 2A6 | 6.2 (I)b | 1.7 ± 0.28 | Mixed (α = 270) | |||
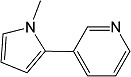 β-Nicotyrine β-Nicotyrine |
2A13 | 8.2 (I) | 0.12 | 5.6 ± 0.86 | Mixed (α = 6.6) | 0.74 |
| 2A6 | 71 (I) | 7.5 ± 2.9 | Mixed (α = 3.5) | |||
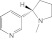 (S)-Nicotine (S)-Nicotine |
2A13 | 54 (I) | 0.11 | 72 ± 4.6 | Competitive | 0.55 |
| 2A6 | 470 (I) | 130 ± 8.8 | Competitive | |||
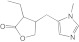 Pilocarpine Pilocarpine |
2A13 | 3.0 (II)c | 0.83 | 1.4 ± 0.12 | Competitive | 0.47 |
| 2A6 | 3.6 (II)c | 3.0 ± 0.45 | Mixed (α = 25) | |||
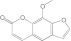 8-MOP 8-MOP |
2A13 | 1.6 (I) | 0.14 | 0.040 ± 0.009 | Mixed (α = 1.9) | 0.16 |
| 2A6 | 11 (I) | 0.25 ± 0.10 | Mixed (α = 3.3) |
The binding modes are shown in parentheses, where (I) represents type I binding and (II) represents type II binding.
Ki ± S.E.
