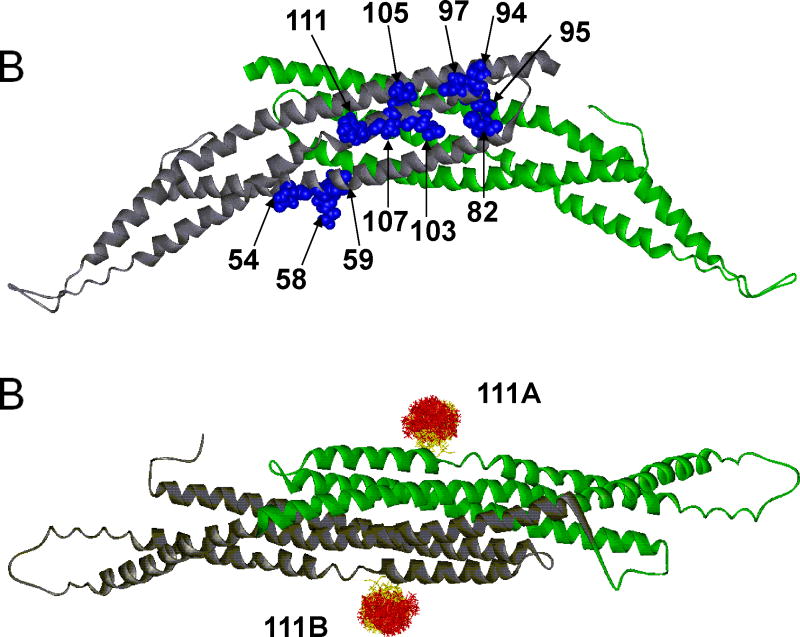
Figure 2.
(A) The 11 labeled sites for the amphiphysin homodimer are indicated in blue in one subunit (which is colored in gray). The second subunit (colored in green) contains the identical sites on the opposite surface of the dimer. The sites are positioned in a region that is largely α-helical. (B) The structure of the amphiphysin dimer is rotated 90° from the position shown in A. An example of spin label distributions calculated by PRONOX is shown for site 111 on each subunit of the dimer (sites 111A and 111B). The label distribution at each site is separated into energetically favorable (yellow) and unfavorable (red) label conformers.