Figure 1.
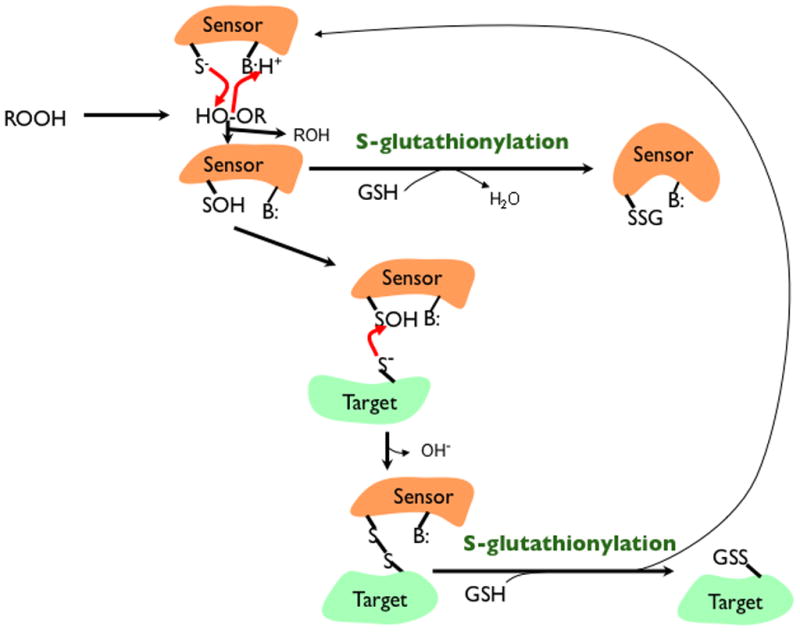
Glutathionylation in signaling. Some proteins, including peroxiredoxins, may be either sensors or direct targets for glutathionylation. When acting as a direct target, glutathionylation would occur on a protein thiolate (S−) after it has been oxidized by a hydroperoxide (ROOH) to a sulfenic acid (SOH) with the assistance of a proton donating amino acid (BH+) that allows ROH to leave. GSH can then react with the SOH; however, either the SOH or GSH would need to be in the anionic form to be a reasonably good nucleophile in that reaction. When acting as a sensor that then assists in glutathionylation, the formation of a disulfide with a target thiolate is a reasonable possibility. This would be followed by disulfide exchange where again the GSH would need assistance from a base (B:) to remove its proton forming GS−.
