Fig. 3.
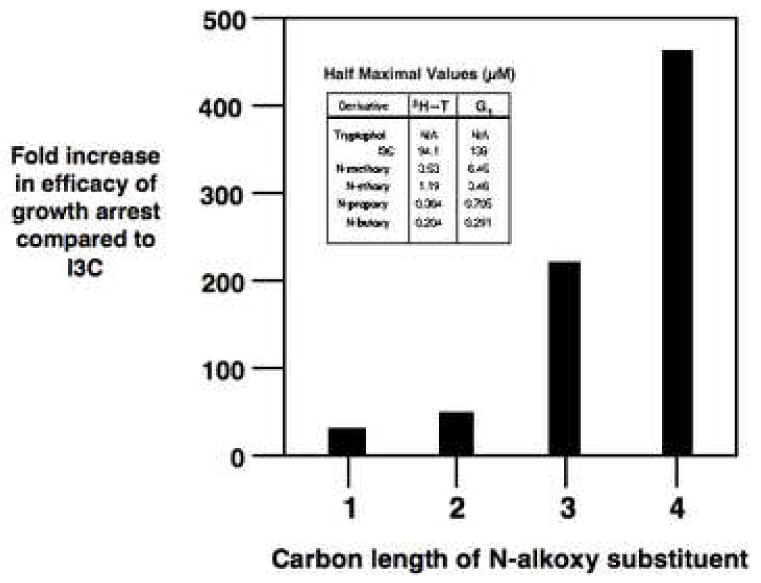
Correlation of the efficacy of the anti-proliferative effects of the N-alkoxy I3C derivatives with the carbon length of the N-alkoxy substituents. The insert shows the indole concentrations for I3C and its N-alkoxy derivatives that induce the half maximal inhibition of DNA synthesis and half maximal G1 cell cycle arrest. The fold efficacy was calculated as the ratio of the half maximal response for each N-alkoxy derivative to the half maximal response observed with the I3C parent compound. The graph correlates the fold efficacy of the growth arrest with the carbon length of the N-alkoxy derivatives. These data were based on three independent DNA synthesis experiments and on three independent determinations of half maximal G1 cell cycle arrest. The approximate standard deviation is −/+ 5% of the final calculated fold efficacies of growth arrest for each N-alkoxy I3C derivative.
