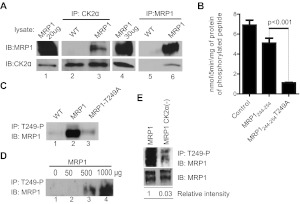
Fig. 5.
A, MRP1 and CK2α proteins interact physically. Protein lysates prepared from WT and MRP1 cells were subjected to CoIP with an antibody against CK2α (goat CK2α; lanes 2 and 3) or an antibody against MRP1 (mouse QCRL-1; lanes 5 and 6). Protein A/G PLUS Agarose was added to precipitate the immunocomplex and proteins were analyzed by immunoblotting (IB). Small aliquot of MRP1 lysate was loaded as a positive control for immunoblotting (lanes 1 and 4). Reactions were carried-out in triplicate and blots shown here are representative of the series. B, phosphorylation of MRP1-Thr249 consensus site by recombinant CK2α is dependent on Thr249. The ability of recombinant human CK2α to phosphorylate MRP1-Thr249 consensus site was assessed by in vitro kinase assay as described under Materials and Methods. Synthetic peptides biotin-Ahx-RRRADDSDDDDDK (Control), biotin-Ahx- LNKEDTSEQVV (MRP1244–255), and biotin-Ahx- LNKEDASEQVV (MRP1244- 254T249A) were incubated in the presence of recombinant human CK2α and 32P-labeled ATP. All experiments were normalized to a zero time point and performed in triplicate, and results are reported as nanomoles of phosphorylated peptide formed per 5 min of reaction per milligram of recombinant protein. Statistical analysis was performed using Student's t test (control peptide served as a positive control to test for activity of the recombinant CK2α protein and therefore was not taken into consideration for statistical analysis). C, MRP1-Thr249-P antibody pulls down MRP1 protein from MRP1 cells but not from MRP1-T249A line. Custom-made rabbit antibody against synthetic peptide corresponding to MRP1-Thr249-P consensus site [WSLNKEDT(p)SEQVVP] was used to immunoprecipitate (IP) MRP1 protein from membrane fractions prepared from WT, MRP1, and MRP1-T249A cells, followed by IB with MRP1 antibody (QCRL-1). D, test of linearity carried out by immunoprecipitation with fixed amount of MRP1-Thr249-P antibody on increasing amount of MRP1 membrane fraction. E, knockdown of CK2α results in decreased MRP1 phosphorylation at Thr249. Membrane fractions prepared from MRP1 and MRP1 CK2α(−) cells were subjected to immunoprecipitation with rabbit MRP1-Thr249-P antibody (top). Relative phosphorylation (phosphorylated MRP1/ total MRP1) was determined by densitometric analysis with use of Adobe Photoshop CS4.