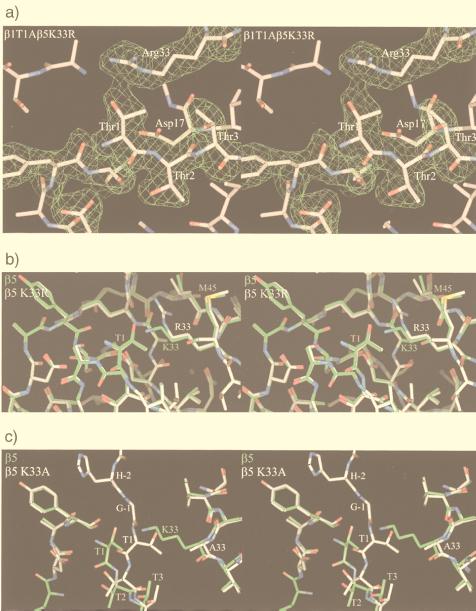
Figure 3.
(a) Stereodiagram of the β1T1A β5K33R double mutant in the vicinity of residue Thr1 in β5. The electron density is calculated with phases from the wild-type β5 model. (b) Stereodiagram of wild-type (green) and β5K33R (white) mutant around Thr1. They superimpose closely except for the site of mutation. β5K33R autolyses and has a free Thr1. (c) Comparison of the wild-type (green) and β5K33A (white) mutant. Loss of the Lys33 side chain leads to a large movement of the backbone of Thr1. The mutant is unable to autolyse and has the propeptide attached.