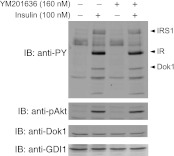
Fig. 7.
YM201636 does not alter tyrosine phosphorylation of Dok1. CHO-T cells were serum-starved for 12 h and incubated for 30 min with vehicle or YM201636 at 160 nM. Cells were then stimulated with insulin (100 nM) for 10 min. Cell lysates were collected in the presence of protease and phosphatase inhibitors. Equal amounts of protein (75 μg) were analyzed by SDS-PAGE and immunoblotting with anti-phosphotyrosine (anti-PY), anti-phosphoThr308-Akt (pAkt), anti-Dok, and anti-GDI1 antibodies (GDI1 is used as a loading control) as indicated, with a stripping step between the antibodies. Shown are chemiluminescence detections of a representative blot out of 3 independent experiments with similar results. Note that the insulin-dependent tyrosine phosphorylation of all phospho-proteins, including Dok1, remains unaltered under these conditions. IRS, insulin receptor substrate.