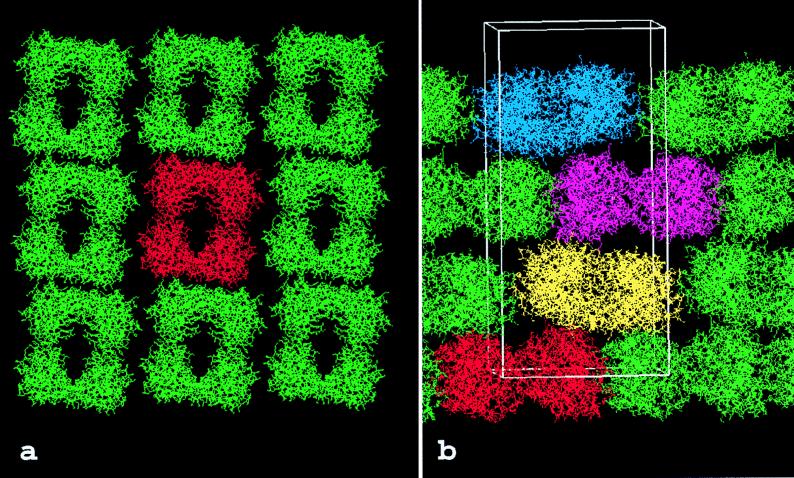
Figure 1.
Packing of the human βII tryptase crystal. (a) View along the z-axis showing one layer of tryptase molecules in the x–y plane. The tryptase monomers are grouped into tetrameric aggregates that form extended sheets. Each of these tryptase tetramers is clearly delimited from its neighbors in both directions. A “reference” tetramer is shown in red for simplicity. (b) View across the z-axis. In the z direction, layers of tetramers are stacked on each other along the 41 screw axis. The local 2-fold symmetry axis is tilted from the z direction by ≈7°, causing increased crystal-stabilizing contacts between layers stacked in the z-direction. One unit cell (82.9 × 82.9 × 172.9Å), occupied by four tryptase tetramers, is indicated by a white bordered box.