Abstract
The human Na+-glucose cotransporter SGLT2 is expressed mainly in the kidney proximal convoluted tubule where it is considered to be responsible for the bulk of glucose reabsorption. Phosphorylation profiling has revealed that SGLT2 exists in a phosphorylated state in the rat renal proximal tubule cortex, so we decided to investigate the regulation of human SGLT2 (hSGLT2) by protein kinases. hSGLT2 was expressed in human embryonic kidney (HEK) 293T cells, and the activity of the protein was measured using radiotracer and whole cell patch-clamp electrophysiology assays before and after activation of protein kinases. 8-Bromo-adenosine cAMP (8-Br-cAMP) was used to activate protein kinase A, and sn-1,2-dioctanoylglycerol (DOG) was used to activate protein kinase C (PKC). 8-Br-cAMP stimulated d-[α-methyl-14C]glucopyranoside ([14C]α-MDG) uptake and Na+-glucose currents by 200% and DOG increased [14C]α-MDG uptake and Na+-glucose currents by 50%. In both cases the increase in SGLT2 activity was marked by an increase in the maximum rate of transport with no change in glucose affinity. These effects were completely negated by mutation of serine 624 to alanine. Insulin induced a 250% increase in Na+-glucose transport by wild-type but not S624A SGLT2. Parallel studies confirmed that the activity of hSGLT1 was regulated by PKA and PKC due to changes in the number of transporters in the cell membrane. hSGLT1 was relatively insensitive to insulin. We conclude that hSGLT1 and hSGLT2 are regulated by different mechanisms and suggest that insulin is an SGLT2 agonist in vivo.
Keywords: protein kinases, insulin, kidney
protein phosphorylation is one of the central mechanisms for short-term regulation of cellular processes (4, 15), and in the last decades the activity of different transport proteins has been shown to be regulated through the action of protein kinases [i.e., Na+-Cl−-glycine (23), Na+-Cl−-GABA (3), organic anion-transporting polypeptide C (24), type II Na+-phosphate (7), Na+-Cl−-taurine (17) cotransporters]. This regulation can be direct or indirect: direct regulation changes the kinetics of the transporter such as substrate affinity and/or turnover number, whereas indirect regulation occurs via modulation of the rate of protein insertion into or retrieval from the membrane.
Na+-dependent glucose transporters (SGLTs) are a wide class of integral membrane proteins that mediate the thermodynamically coupled transport of sugars and Na+ across different epithelial cells (for an extensive review see Ref. 28). The family includes six different members (SGLT1–6) with different substrate specificity and tissue localization (29). SGLT1 is expressed mainly in the intestine, whereas SGLT2 is predominantly expressed in the kidney where it is involved in the bulk of glucose reabsorption from the glomerular filtrate (26). Biophysical characterization of hSGLT2 expressed in human embryonic kidney cells (HEK 293T) showed that hSGLT2 displays similar glucose and sodium kinetics to hSGLT1 but with a lower concentrative power due to a Na+-glucose coupling of 1:1 rather than 2:1 for SGLT1 (13). Studies have shown that both protein kinase A (PKA) and protein kinase C (PKC) are involved in the regulation of SGLT1-mediated sugar transport (27). Regulation occurs by modulation of the rate of exo- and endocytosis and not directly by phosphorylation of the protein (27).
A recent phosphoproteomic analysis of membrane proteins in the rat renal cortex has shown that SGLT2 exists in a phosphorylated state (6). This led to the identification of three candidate phosphorylation sites: Ser619, Ser621, and Ser623 in the internal loop between transmembrane domain (TM) 12 and 13. To test whether phosphorylation is critical in the regulation for the human SGLT2, we have analyzed the effect of PKA and PKC activation on hSGLT2 expressed in HEK-293T cells. Parallel experiments were performed on hSGLT1 to highlight any potential differences between the two isoforms. PKA activation was induced by 8-bromoadenosine 3′-5′-cAMP (8-Br-cAMP), whereas sn-1,2-dioctanoylglycerol (DOG) was used as the activator of the Ca2+/ test whether regulation of hSGLT2 occurs via phosphorylation, we also studied a mutant of the putative phosphorylation sites. Finally, since SGLT2 is highly expressed in the proximal tubule where there is a high density of insulin binding sites (2, 25), we examined whether or not insulin could be a natural agonist of SGLT activity.
Our results demonstrate that PKC and PKA regulate hSGLT2 activity in HEK-293T cells by phosphorylation versus indirect regulation of SGLT1, and that insulin may be the physiological agonist regulating SGLT2 activity in vivo.
MATERIALS AND METHODS
Reagents and solutions.
The standard extracellular solution (Na+ buffer) contained (in mM) 150 NaCl, 1 CaCl2, 1 MgCl2, and 10 HEPES, pH 7.4. For Na+-free solution (choline buffer) NaCl was equimolarly replaced with choline Cl. For whole cell patch-clamp experiments at 37°C, mannitol (100 mM) was added to the extracellular solution to reduce noise and improve stability in the whole cell recording mode. The standard intracellular solution (pipette solution) contained (in mM) 145 CsCl, 5 NaCl, 11 EGTA, and 10 HEPES, pH 7.4.
8-Br-cAMP and DOG were purchased from Sigma (St. Louis, MO) and Calbiochem-Novabiochem (La Jolla, CA). Insulin and insulin-like growth factor (IGF1) were purchased from Sigma.
Molecular biology.
The S624A mutant was generated using the Quickchange site-directed mutagenesis kit from Stratagene (Agilent Technologies, Santa Clara, CA). The fidelity of the mutagenesis was confirmed by sequencing the entire coding region (GenoSeq, UCLA, CA).
Cell culture and transfection.
HEK-293T cells were purchased from the American Type Culture Collection (Manassas, VA). Cells were grown in vented 25-cm2 polystyrene flasks in Dulbecco's modified Eagle's medium (DMEM, CELLGRO, Manassa, VA) supplemented with 10% fetal (Valley Biomedical Products, Winchester, VA) and 1% penicillin-streptomycin (Invitrogen, Carlsbad, CA). Cells were maintained in incubator at 37°C in humidified atmosphere of 5% CO2 and 95% air. Cells were passaged (1:10) every 5 days, and before transfection, they were seeded in six-well plates or poly-l-lysine-coated 24-well plates. Cells were grown to 50–70% confluence and then transfected using the Effectene kit (Quiagen, Germantown, MD) following protocol instructions as already described elsewhere (13).
d-[α-Methyl-14C]glucopyranoside uptake.
Sugar uptake was measured in HEK-293T cells expressing hSGLT1, hSGLT2 or hSGLT2 S624A mutant as already described (13). In brief, cells were incubated at 37°C for 30 min in 50 μM d-[α-methyl-14C]glucopyranoside (α-MDG, GE HealthCare Life Sciences, Piscataway, NJ), a specific substrate for SGLTs, and washed in cold choline buffer three times, and a cell sample was assayed using scintillation counting. For each tested condition, the sample size was n = 3–4 wells, and each experiment was repeated at least twice. Uptakes were measured in the presence and absence of 100 μM phlorizin, and the SGLT-specific uptake was taken as the phlorizin-sensitive component (13).
Whole cell patch-clamp recording.
Our procedure for whole cell patch-clamp experiments has been explained elsewhere (13). In brief, experiments were performed 2 days after transfection. The same day cells were plated on 12-mm poly-l-lysine-coated glass coverslips and selected on the basis of fluorescence intensity using a Nikon diaphot epifluorescence microscope (Nikon, Tokyo, Japan). During the experiment cells were kept at the holding potential (Vh)= −60 mV and gravity perfused using a set of 0.25-mm cannulas positioned near the cell. For hSGLT2 experiments, solutions were heated at 37°C via an in-line solution heater (TC-324B Werner Instrument), whereas for hSGLT1, experiments were carried out at 22°C. For recordings at constant holding potential, currents were filtered at 2 kHz and digitized at 1 kHz.
Steady-state kinetics were determined using a voltage-step protocol. The membrane voltage was stepped from Vh = −60 mV to the test potential ranging between −150 mV and in +50 mV in 20-mV increments for a duration of 100 ms and averaged over four sweeps. In this case the current was filtered at 2 kHz and digitized at 50 kHz.
Steady-state glucose currents (IGlu) were obtained by subtracting the baseline current recorded in Na+ buffer to the total current measured in Na+ buffer +Glu:
Steady-state glucose activation was determined by varying the glucose concentration in the presence of Na+ buffer. Glucose response to a change in substrate concentration was fitted with the modified Hill equation of the form
where Imax is the maximal current, [S]o is the external substrate concentration, and K0.5 is the substrate concentration at half-maximal current.
Activation of protein kinases by 8-Br-cAMP or DOG.
The approach used to activate PKA and PKC was that previously described for hSGLT1 in Xenopus laevis oocytes (11). 8-Br-cAMP was added to Na+ buffer from a 0.1 M stock (in water, stored at −20°C) to give a final concentration of 0.1 mM. DOG was added from a 1 mM stock (in water, stored at −20°C) to give a final concentration of 1 μM. For uptake experiments, cells were preincubated in medium containing DOG or cAMP for the indicated time at 37°C. After the preincubation time, cells were washed with PBS three times and uptake was then performed as described before. For patch-clamp experiments each coverslip was divided in two halves. Cells from one half were measured in control conditions while the other half was preincubated in 8-Br-cAMP or DOG for the indicated time, and then glucose currents were measured. This protocol was predicated by the technical difficulties in holding cells in the whole cell patch-clamp mode at 37°C for lengthy periods of time.
Insulin activation.
Insulin was added from a 100 μM stock (in water, stored at 4°C) to give final concentrations of 100 and 400 pM. Insulin was added to complete DMEM medium, and cells were incubated for 2 h at 37°C and controlled O2-CO2 before the transport assay. Uptake was then measured according to the protocol already described. Insulin-like growth factor was added from a 100 μg/ml stock (in water, stored at 4°C) to give the final concentration of 100 ng/ml. Cells were incubated for 2 h in PBS at 37°C and controlled O2-CO2, and uptake was then measured according to protocols already described.
Statistical analysis.
Statistical analysis was made using GraphPad Prism version 4.02 for Windows, GraphPad Software. Results are provided as means ± SE. All data were tested for significance using the one-way ANOVA and Turkey post test. Values of P < 0.05 were considered significant.
RESULTS
8-Br-cAMP increased [14C]-αMDG uptake and glucose-induced current in cells expressing hSGLT2.
To test whether activation of protein kinases is able to modulate the transport activity of hSGLT2, we compared radiotracer uptakes before and after incubation with 8-Br-cAMP, a PKA activator (10) or DOG, an activator of PKC (20). As a control we performed parallel experiments on hSGLT1 since it was previously shown (11) that in X. laevis oocytes the activation of PKA or PKC induces a 30% increase in the maximal transport activity.
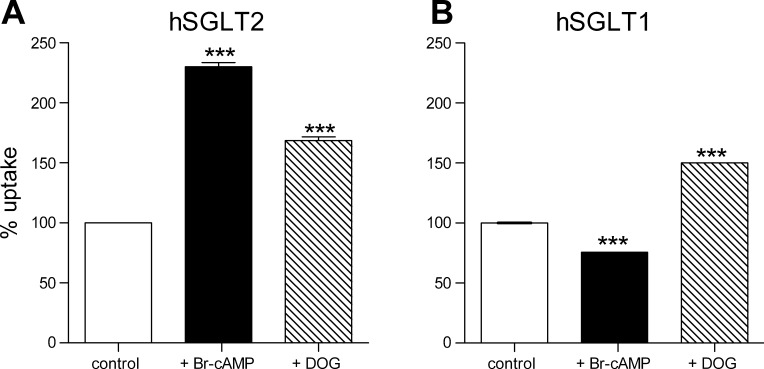
Figure 1A shows that 1 h incubation in 8-Br-cAMP and DOG increased radiotracer uptake by 225% and 150% in cells expressing SGLT2. In cells expressing hSGLT1 8-Br-cAMP inhibited uptake by 25% while DOG increased uptake by 49%. There was no effect of either 8-Br-cAMP or DOG on α-MDG uptake into nontransfected HEK-293T cells (not shown).
Fig. 1.
Stimulation of PKA or PKC affects d-[α-methyl-14C]glucopyranoside (α-MDG, 50 μM) uptake from hSGLT1- and hSGLT2-expressing cells. A: uptake measured from human embryonic kidney (HEK)-293T cells expressing hSGLT2 before and after incubation with 0.1 mM 8-bromo-adenosine cAMP (8-Br-cAMP) or 1 μM sn-1,2-dioctanoylgly (DOG) for 1 h. Uptake is normalized for the picomoles per minute per microgram obtained in control condition. n = 6 wells from 2 different experiments. Error bars are means ± SE. ***P ≤ 0.001. B: uptake was measured from HEK 293T cells expressing hSGLT1 before and after incubation (1 h at 37°C) with 8-Br-cAMP or DOG. Uptake is normalized for the picomoles per minute per microgram obtained in control condition. n = 9 wells from 3 different experiments. Error bars are means ± SE. ***P ≤ 0.001.
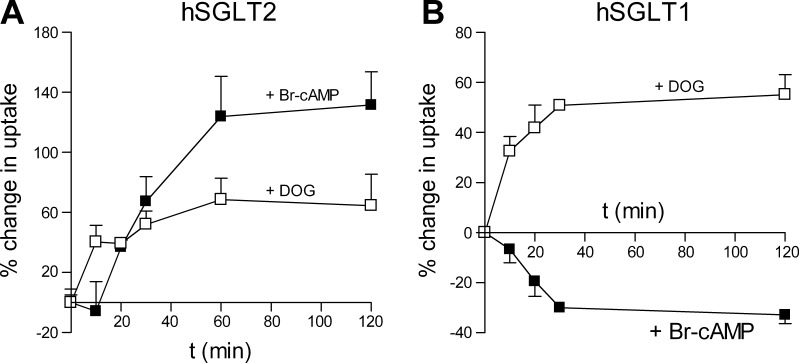
To further characterize the effect of activation of PKA and PKC on hSGLT2 and hSGLT1, we measured the time dependence of activation by incubating cells for 10, 20, 30, 60, and 120 min with the two compounds and then measuring radiotracer uptake. For hSGLT2, 8-Br-cAMP activation required at least 15 min since uptakes at 10 min were not significantly different from cells not exposed to the reagent (Fig. 2A). The full response was observed with 60-min incubations. For hSGLT1 significant changes in uptake were observed after 10 min for 8-Br-cAMP and DOG, and full responses were obtained within 30 min (Fig. 2B).
Fig. 2.
Time course of 8-Br-cAMP and DOG effects on hSGLT1 and hSGLT2 transport rate. A: uptake from hSGLT2-transfected cells was measured in control condition and after 10, 20, 30, 60, and 120 min incubation in 8-Br-cAMP (solid squares) or DOG (open squares). Each time point is the mean of at least 6 wells measured from 2 different experiments. Data points are joined only for visualization. Error bars are means ± SE. B: uptake from hSGLT1-transfected cells was measured in control condition and after 10, 20, 30, 60, and 120 min incubation in 8-Br-cAMP (solid squares) or DOG (open squares). Each time point is the result of at least 6 wells measured from 2 different experiments. Data points are joined only for visualization. Error bars are means ± SE.
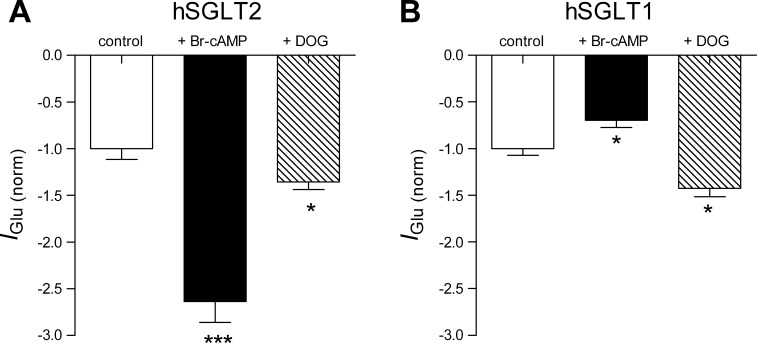
Both hSGLT1 and hSGLT1 are electrogenic transport proteins (28), so we exploited this property to test the effect of 8-Br-cAMP and DOG on glucose-induced current (IGlu) under voltage-clamp conditions. Figure 3A shows that 8-Br-cAMP increased the hSGLT2 Na+-glucose inward current by 250% at the Vh of −60 mV, whereas DOG produce a small significant increase (23%). For hSGLT1 8-Br-cAMP decreased the inward current by 33 ± 5%, whereas DOG decreased it by 49 ± 2% (Fig. 3B).
Fig. 3.
Activation of PKA and PKC changes the transport rate of hSGLT1 and hSGLT2. A: glucose-induced current measured from hSGLT2 cells at holding potential (Vh) = −60 mV before and after incubation with 8-Br-cAMP or DOG for 1 h. Whole cell patch-clamp recordings were performed at 37°C. Data points are mean from at least 11 cells ± SE. ***P ≤ 0.001, *P ≤ 0.05. B: glucose-induced current from hSGLT1 was measured at Vh = −60 mV before and after incubation with 8-Br-cAMP or DOG for 1 h. n = 6 from different experiments. Data are means ± SE. *P ≤ 0.05.
PKA and PKC increase hSGLT2 maximum rate of transport without altering the kinetic properties.
Protein kinases regulate the activity of membrane transport proteins either directly or indirectly. Direct effects occur through the phosphorylation of the protein and may lead to a change in the kinetics of the membrane transport such as substrate affinity and/or turnover rate. Indirect regulation alters the rate of insertion into or retrieval from the plasma membrane.
We have used electrophysiological techniques to discriminate between these two possibilities: 1) substrate-induced currents are used to determine substrate affinity (K0.5) and maximum rate of transport (Imax); and 2) capacitance measurements (Cm) give information about the area of the plasma membrane and the changes in area are associated with vesicle exocytosis or endocytosis (11). We divided each coverslip of SGLT-transfected cells into two parts: we measured on one half IGlu in control cells and on the other half cells exposed to 8-Br-cAMP or DOG for 1 h (Owing to technical difficulties with whole cell patch experiments at 37°C and the time required to active PKA and PKC, it was not possible to study the effects of 8-Br-cAMP or DOG on a single cell).
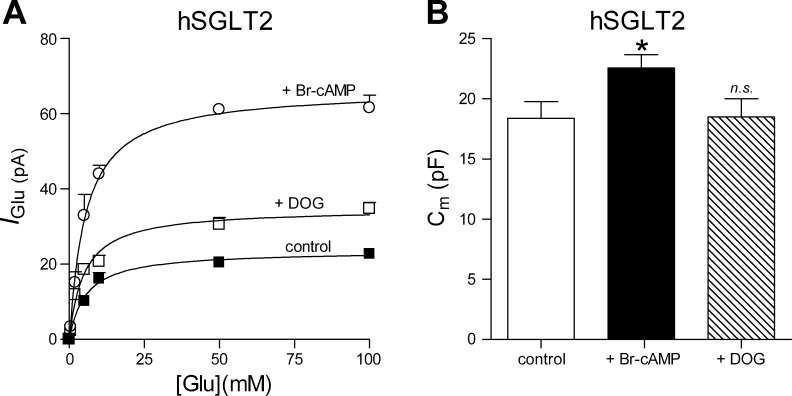
As shown in Fig. 4A the glucose-induced current was measured as a function of external glucose concentration up to 100 mM before and after incubation with 8-Br-cAMP or DOG. Data obtained from different cells from the same transfection day were pooled, and the Imax and K0.5Glu were obtained for the three different conditions. Imax was 23.5 ± 1.7 pA in control conditions (Fig. 4A, solid squares), 66 ± 4 after incubation in 8-Br-cAMP (open circles), and 35 ± 2 after DOG (open squares). We did not detect any effect on the glucose apparent affinity K0.5Glu: 5.4 ± 1.3, 5.0 ± 0.8, and 5.2 ± 0.9 mM for controls, 8-Br-cAMP (Fig. 4A, open circles), and DOG (open squares), respectively. To test whether activation of PKA and PKC is associated with an increase in the plasma membrane area, we measured membrane capacitance. As shown in Fig. 4B activation of PKA resulted in a significant increase in Cm (from 18 pF ± 1 to 23 pF ± 1), suggesting an increase of the rate of vesicle fusion with the membrane. Activation of PKC did not result in any significant change of the membrane capacitance.
Fig. 4.
Changes in the apparent glucose affinity and plasma membrane area for hSGLT2 caused by effectors of intracellular signaling pathway. A: glucose-induced current was measured upon addition of 0–100 mM glucose at a Vh = −60 mV before and after incubation with the protein kinases activators. Data points were fitted with Eq. 2 to get the apparent glucose affinity (K0.5Glu). Each data point is the mean of n ≥ 5. Data are means ± SE. B: membrane capacitance (Cm) measured before and after incubation with PKA and PKC activator. Values are means of 11 cells ± SE. *P ≤ 0.05.
Published results (11) indicate that SGLT1 regulation in X. laevis oocytes occur via modulation of the rate of insertion into or retrieval from the membrane. Protein trafficking of the SGLT1 can be monitored as a change in SGLT1 capacitance currents (Q) and Cm, so we decide to perform parallel experiments on cells expressing hSGLT1. In accordance with the change in Imax we observed that 8-Br-cAMP decreased Qmax and Cm by 20 ± 4% and DOG increased both by 33 ± 1%.
Protein kinases activation is due to direct phosphorylation of hSGLT2 and is stimulated by insulin.
Our results indicate that PKA and PKC activation induces an increase in hSGLT2 Imax and Cm indicating an increase in the catalytic turnover of existing SGLT2 proteins in the plasma membrane and/or increase in the number of proteins in the membrane. Is this activation due to the direct phosphorylation of hSGLT2? To answer this question we decide to mutate phosphorylation consensus sites and test whether PKA and PKC activation can still modulate SGLT2 transport.
A recent study (6) showed that the rat hSGLT2 displays three candidate phosphorylation sites (Ser619, Ser621, and Ser623) in the loop between transmembrane domain (TM) 12 and 13. When we aligned the rat SGLT2 with the human sequence we found that only Ser623 is conserved and corresponds to Ser624 in the human sequence. We therefore mutated Ser624 to an alanine and we tested the effect of activation of PKA or PKC.
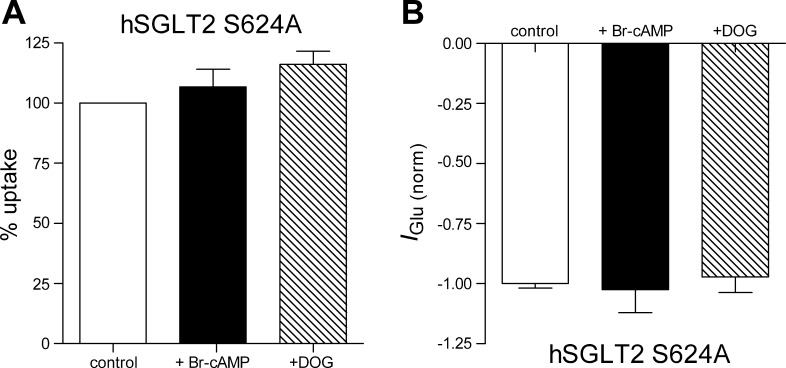
After expression in HEK-293T cells, the S624A mutant gave a robust [14C]α-MDG uptake that is indistinguishable from the uptake from hSGLT2 wt (data not shown). This suggests that the mutant protein is functional and that Ser624 is not a critical residue. As shown in Fig. 5A for the S624A SGLT2 mutant, there was no effect of 8-Br-cAMP or DOG on [14C]α-MDG uptake. We performed parallel electrophysiological experiments under voltage-clamp condition. As observed for radiotracer uptake experiments, there was no effect of PKA or PKC activation on the glucose-induced currents (Fig. 5B).
Fig. 5.
Serine 624 removal prevents the PKA and PKC stimulation. A: d-[α-methyl-14C]glucopyranoside uptake form hSGLT2 wild-type and S624A mutant before and after incubation with 8-Br-cAMP and DOG for 1 h. Bars are means of 6 wells from 2 different experiments ± SE. B: glucose-induced current measured from cells expressing hSGLT2 wild-type or S624A mutant. Values are means of 6 cells ± SE.
Glucose plasma concentration is regulated by different hormones, but one of the best known is insulin that is secreted after a meal to reduce the level of circulating glucose. In the kidney the highest density of insulin binding coincides with the location of SGLT2 expression, the proximal tubule (2, 25), and so we decided to test if insulin can modulate hSGLT2 activity in HEK 293T cells.
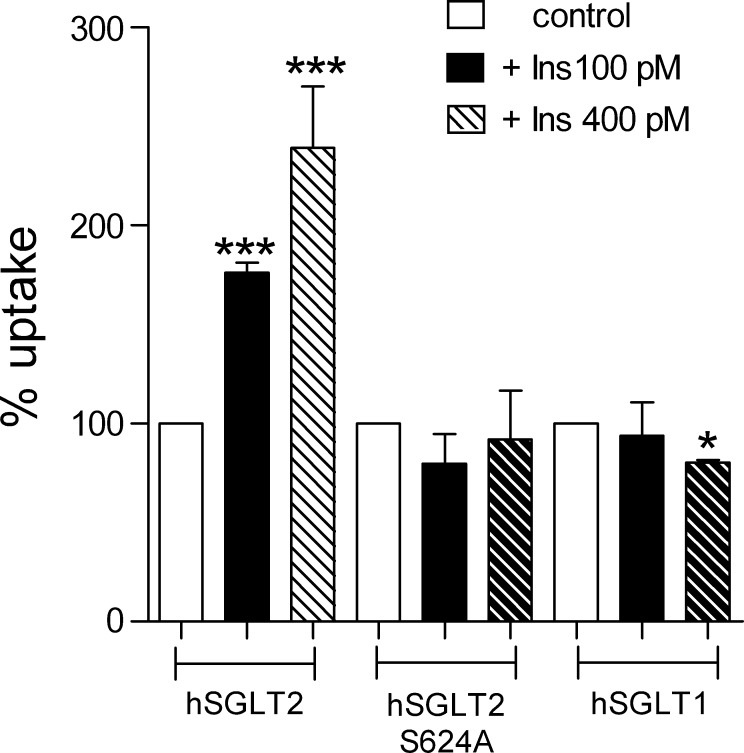
Cells were incubated 2 h in 100 or 400 pM insulin, and [14C]α-MDG uptake was measured in control condition and after insulin treatment. As shown in Fig. 6, 100 pM insulin induce a 72% increase in the uptake, whereas higher concentration (400 pM) induced a 2.3-fold increase. To test whether insulin activation occurs via protein phosphorylation, we tested the effect of insulin in cells expressing the hSGLT2-S624A mutant. As shown in Fig. 6, removal of Ser264 was sufficient to completely abolish the insulin response. The specificity of the insulin effect was determined by conducting parallel experiment with hSGLT1; no effect was observed with 100 pM insulin but a 20% decrease was observed with 400 pM (Fig. 6).
Fig. 6.
Insulin effect on hSGLT2 wild-type and hSGLT2 S524A mutant. d-[α-methyl-14C]glucopyranoside uptake measured in control condition (open bars) and after preincubation for 2 h in DMEM supplemented with 100 pM (black bars) or 400 pM insulin (hatched bars) at 37°C from hSGLT2, hSGLT2 S624A, and hSGLT1-transfected cells. n ≥ 8 wells from at least 2 different experiments. Values are means ± SE. ***P ≤ 0.001 *P ≤ 0.05.
Transcriptosome analyses of the rat renal proximal tubule (12) have shown that proximal tubule cells express the insulin-like growth factor 1 receptor (IGF-1) but not the insulin receptor. We tested whether the insulin stimulation of SGLT2 activity in HEK-293T cells is mediated through the IGF-1 receptor by incubating cells in 100 ng/ml IGF-1. There was no significant difference in glucose transport between the IGF-1-treated and untreated control cells (data not shown).
DISCUSSION
The kidney plays a central role in the regulation of glucose homeostasis by reabsorbing all the 180 g of glucose that is filtered in the glomerulus. SGLT2, localized in the S1 and S2 segments of the proximal tubule, is responsible for the reuptake of 80–90% of the glucose load, whereas SGLT1, localized in the more distal parts of the proximal tubule, reabsorbs the remaining 10–20% (26).
Interest in the regulation of SGLT2 activity in the kidney has increased, especially given recent evidence that SGLT2 in the rat renal cortex is phosphorylated (6). In the present study we analyzed the effect of PKA and PKC on the regulation of the human sodium-dependent glucose cotransporter hSGLT2. Parallel experiments were performed on human SGLT1 since in a previous study it was shown that, in X. laevis oocytes, it is regulated by activation of PKA or PKC (11). Both SGLT proteins were expressed in HEK-293T cells, and we monitored glucose transport by radiotracer uptake or whole cell patch-clamp Na-glucose currents before and after incubation with the well-known PKA and PKC activators. 8-Br-cAMP has long been used to activate PKA in oocytes or cells expressing cloned membrane proteins (i.e., Refs. 1 and 23), and the phorbol ester DOG have been used to activate PKC (i.e., Refs. 3 and 9). Both compounds are membrane-permeable reagents and act on the last step in the activation of the protein kinases.
This study demonstrates that hSGLT2, like hSGLT1, is upregulated by activation of PKA and PKC. Radioactive glucose uptakes and glucose-induced currents measured in the human cells transfected with hSGLT2 were increased 200% by activation of PKA and stimulated 50% by activation of PKC (Fig. 1A). Similar results were obtained with hSGLT2 expressed in Chinese hamster ovary (CHO) cells (data not shown). As observed in X. laevis oocytes, hSGLT1 is upregulated by PKC in fact both glucose uptakes and Imax are increased 50%. However, unlike oocytes, hSGLT1 activity in HEK-293T cells was inhibited 24% by activation of PKA (Fig. 1B). The discrepancy between the results in oocytes and HEK-293T cells is most likely due to the differences between the cell types. This indicates that caution should be exercised in extrapolating results obtained on complex signaling networks in model systems to whole animals. The changes in SGLT transport activity that we observed occurred within minutes and were fully reversible (data not shown). No effect of 8-Br-cAMP or DOG was observed on α-MDG uptake in untransfected HEK-293T cells indicating that the protein kinases specifically regulate the expression of the SGLT sugar transporters.
The observed changes in the Imax of hSGLT1 and hSGLT2 could be the result of two different processes: 1) direct change of the activity of the protein in the plasma membrane due to a change in the catalytic rate; or 2) a rapid change in the number of transporters in the plasma membrane due to fast recruitment from an intracellular pool (11). To discriminate between these two possibilities we took advantage of our biophysical assays to measure the density of SGLT proteins in the plasma membrane (Qmax) and/or changes in membrane area caused by vesicle insertion or retrieval.
Charge movements (Q) induced by voltage jumps are a common property of voltage-sensitive membrane proteins such as SGLT1 (11) and other electrogenic Na+-dependent cotransporters (i.e., Refs. 8, 21, and 22). They appear as relaxations in the range of milliseconds superimposed on the cell capacitive-charging transient. From analysis of the transient charge movements the number of transporters present in the membrane can be obtained from the maximal moved charge (Qmax), and the turnover rate can be estimated from the ratio Imax/Qmax (11). Furthermore, any rapid change in the number of SGLT proteins in the plasma membrane due to PKA and PKC activation may also be followed by changes in membrane area (membrane capacitance Cm) due to alteration in rates of endocytosis and/or exocytosis.
As already observed for hSGLT1 expressed in X. laevis oocytes (11), we observed that PKC and PKA activation resulted in fast changes in maximum rates of transport without any effect on the apparent affinity for glucose. Similar results were obtained also for the Na+-Cl−-taurine transporters (17) and Na+-Cl−-GABA cotransporters (3, 16) expressed in X. laevis oocytes and Na+-Cl−-glycine transporters expressed in HEK-293 cells (23). These changes in maximum transport capacity of hSGLT1 were associated with changes in the number of transporters in the Qmax and Cm indicating changes in the rate of exocytosis or endocytosis of SGLT1.
For technical reasons in this study we were unable to resolve hSGLT2 charge movements (Q), but Cm measurements indicate the 8-Br-cAMP activation was associated with a significant increase in membrane area (Fig. 4B). This suggests that regulation of hSGLT2 by PKA may be in part due to recruitment from an intracellular pool of SGLT2 vesicles to the plasma membrane. We could not detect any significant change in the Cm measured from DOG-treated cells (Fig. 4B). The increase in the Cm is due to the balance between increased rate of exocytosis and rate of endocytosis, so a possible explanation for the lack of effect of DOG on Cm is that the increase in exocytosis rate is not enough to overcome the endocytotic rate.
Since we cannot completely exclude that the increase in Imax is associated with a change in the Qmax or in the turnover rate, we decide to test whether PKA/PKC stimulation is due to the direct phosphorylation of the protein. In a recent study (6), the phosphoproteomic analysis of membrane proteins in the rat renal proximal and distal tubule has revealed that rSGLT2 is phosphorylated. Three phosphorylation sites, localized in the loop between transmembrane segments 12 and 13, have been identified: Ser619, Ser621, and Ser623.
Analysis of the human SGLT2 sequence showed that only one of the three phosphorylated serines is conserved (Ser624 corresponding to Ser623 in rat SGLT2), and so we mutated this residue to alanine and tested whether activation of PKA/PKC is still able to stimulate glucose transport. Removal of serine does not impair normal glucose transport; there was no change in sugar uptake or Imax, indicating that this residue is not important for the proper transport function. However, the upregulation of S624A SGLT2 by PKA and PKC protein kinases was completely absent (Fig. 5). This strongly suggests that the PKA and PKC act through different pathways to phosphorylate S623 and stimulate glucose transport. Interestingly, S624 does not belong to any known consensus phosphorylation site.
Given that hSGLT2 is regulated by protein kinases the immediate question becomes what agonists are responsible. Major clues are that insulin appears to regulate glucose reabsorption in the kidney, at least in a chronic fashion in diabetes. The maximum tubular glucose reabsorption is increased in diabetic subjects, and this was reduced by insulin (5, 19, 18). Furthermore, there is a high density of insulin binding to the rat proximal convoluted tubule that coincides with expression of SGLTs (2, 25). We then decided to test the acute effects of insulin on the activity of SGLT1 and SGLT2 expressed in HEK-293T cells. At two insulin concentrations corresponding to those recorded after a carbohydrate meal, 100 and 400 pM, there was a dramatic increase in Na-glucose transport by SGLT2 but only a modest reduction in transport by SGLT1 at the higher concentration. In the case of the S624A SGLT2 mutant, insulin activation was completely suppressed suggesting that insulin acts through phosphorylation of this residue. The lack of effect of insulin-like growth factor 1 (IGF-1) on transport activity of hSGLT2 expressed in HEK-293T cells, moreover, indicates that the insulin effect is mediated by the insulin receptor and not the IGF-1 receptor. At this time we have no information about the signaling cascades between the insulin receptor and phosphoryation of S624 SGLT2.
Our results suggest that hSGLT2 activity in the proximal tubule may be regulated over the short term by insulin, and this may be part of a physiological response following carbohydrate-rich meals to ensure complete glucose reabsorption in the kidney. In the fasting state the blood glucose is low (4 mM), but after a carbohydrate meal this increases rapidly to 7–8 mM. The accompanying pancreatic insulin secretion increases the plasma insulin concentration from 40 to 400 pM which returns the elevated blood glucose to the fasting level. The increase in plasma insulin is expected to increase SGLT2 activity in the proximal tubule, and this will ensure that glucose is not lost to the urine. In diabetic subjects there is a reduction in pancreatic insulin secretion after a meal, and so we expect a loss of SGLT2 upregulation that would contribute to the diabetic glucosuria. In vivo the SGLT2 response to insulin may not be mediated by the insulin receptor but by the IGF-1 receptor; analyses of the data available in the rat transcriptosome databases indicates that the IGF-1 receptor, and not the insulin receptor, is expressed in the proximal tubule (12). Both are close members of the tyrosine kinase family of receptors activated by insulin (14). In HEK-293T cells IGF-1 does not mimic the insulin effect of hSGLT2 activity.
In summary, glucose transport by hSGLT2 is dramatically upregulated by activation of PKA and PKC when expressed in the human cell line HEK-293T and that an agonist for this affect in vivo may be insulin. These effects are mediated by phosphorylation of S624 in hSGLT2. We speculate that the insulin regulation of SGLT2 in vivo plays a part in the renal glucose reabsorption after a meal, and that the lack of this regulation contributes to the renal glucosuria in diabetic patients.
GRANTS
Our study was supported by a Fellowship from the Swiss National Science Foundation PBZHP3-135919 (to C. Ghezzi) and grants from the National Institutes of Health (DK-19567 and DK-77133).
DISCLOSURES
E. M. Wright serves on an SGLT2 Inhibitor Advisory Board for Boehringer-Ingelheim and has been a consultant for BMS/AZ, Roche, Merck, GSK, and Novartis on SGLT biology, and been a speaker on SGLT biology at sponsored workshops and symposia.
AUTHOR CONTRIBUTIONS
Author contributions: C.G. and E.M.W. conception and design of research; C.G. performed experiments; C.G. and E.M.W. analyzed data; C.G. and E.M.W. interpreted results of experiments; C.G. and E.M.W. prepared figures; C.G. and E.M.W. drafted manuscript; C.G. and E.M.W. edited and revised manuscript; C.G. and E.M.W. approved final version of manuscript.
ACKNOWLEDGMENTS
It is a pleasure to acknowledge our debt to Drs. B. Hirayama, C. Hummel, and D. Loo, Dept. of Physiology, Geffen School of Medicine at UCLA, for their support during all phases of this project. We also acknowledge the Reviewers for their constructive criticisms, especially for bringing to our attention that the IGF-1 receptor may mediate the insulin response in the rat proximal tubule.
REFERENCES
- 1. Blumenthal EM, Kaczmarek LK. Modulation by cAMP of a slowly activating potassium channel expressed in Xenopus oocytes. J Neurosci 12: 290–296, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Butlen D, Vadrot S, Roseau S, Morel F. Insulin receptors along the rat nephron: [125I] insulin binding in microdissected glomeruli and tubules. Pflügers Arch 29: 325–332, 1988 [DOI] [PubMed] [Google Scholar]
- 3. Corey JL, Davidson N, Lester HA, Brecha N, Quick MW. Protein kinase C modulates the activity of a cloned gamma-aminobutyric acid transporter expressed in Xenopus oocytes via regulated subcellular redistribution of the transporter. J Biol Chem 269: 14759–14767, 1994 [PubMed] [Google Scholar]
- 4. Edelman AM, Blumenthal DK, Krebs EG. Protein serine/threonine kinases. Annu Rev Biochem 56: 567–613, 1987 [DOI] [PubMed] [Google Scholar]
- 5. Farber SJ, EYB DPE. Effect of diabetes and insulin of the maximum capacity of the renal tubules to reabsorb glucose. J Clin Invest 30: 125–129, 1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feric M, Zhao B, Hoffert JD, Pisitkun T, Knepper MA. Large-scale phosphoproteomic analysis of membrane proteins in renal proximal and distal tubule. Am J Physiol Cell Physiol 300: C755–C770, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Forster IC, Traebert M, Jankowski M, Stange G, Biber J, Murer H. Protein kinase C activators induce membrane retrieval of type II Na+-phosphate cotransporters expressed in Xenopus oocytes. J Physiol 517: 327–340, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Forster IC, Wagner CA, Busch AE, Lang F, Biber J, Hernando N, Murer H, Werner A. Electrophysiological characterization of the flounder type II Na+/Pi cotransporter (NaPi-5) expressed in Xenopus laevis oocytes. J Membr Biol 160: 9–25, 1997 [DOI] [PubMed] [Google Scholar]
- 9. Hayes G, Busch AE, Lang F, Biber J, Murer H. Protein kinase C consensus sites and the regulation of renal Na/Pi-cotransport (NaPi-2) expressed in Xenopus laevis oocytes. Pflügers Arch 430: 819–824, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Hei YJ, MacDonell KL, McNeill JH, Diamond J. Lack of correlation between activation of cyclic AMP-dependent protein kinase and inhibition of contraction of rat vas deferens by cyclic AMP analogs. Mol Pharmacol 39: 233–238, 1991 [PubMed] [Google Scholar]
- 11. Hirsch JR, Loo DD, Wright EM. Regulation of Na+/glucose cotransporter expression by protein kinases in Xenopus laevis oocytes. J Biol Chem 271: 14740–14746, 1996 [DOI] [PubMed] [Google Scholar]
- 12. Huling JCPT, Song JH, Yu MJ, Hoffert JD, Knepper MA. Gene expression databases for kidney epithelial cells. Am J Physiol Renal Physiol 302: F401–F407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hummel CS, Lu C, Loo DD, Hirayama BA, Voss AA, Wright EM. Glucose transport by human renal Na+/d-glucose cotransporters SGLT1 and SGLT2. Am J Physiol Cell Physiol 300: C14–C21, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim JJ, Accili AD. Signalling through IGF-I and insulin receptors: where is the specificity? Growth Horm IGF Res 12: 84–90, 2002 [DOI] [PubMed] [Google Scholar]
- 15. Krebs EG, Beavo JA. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem 48: 923–959, 1979 [DOI] [PubMed] [Google Scholar]
- 16. Loo DD, Hazama A, Supplisson S, Turk E, Wright EM. Relaxation kinetics of the Na+/glucose cotransporter. Proc Natl Acad Sci USA 90: 5767–5771, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Loo DD, Hirsch JR, Sarkar HK, Wright EM. Regulation of the mouse retinal taurine transporter (TAUT) by protein kinases in Xenopus oocytes. FEBS Lett 392: 250–254, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest 28: 101–109, 1971 [DOI] [PubMed] [Google Scholar]
- 19. Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 54: 3427–3434, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Ruth P, Landgraf W, Keilbach A, May B, Egleme C, Hofmann F. The activation of expressed cGMP-dependent protein kinase isozymes I alpha and I beta is determined by the different amino-termini. Eur J Biochem 202: 1339–1344, 1991 [DOI] [PubMed] [Google Scholar]
- 21. Sacher A, Nelson N, Ogi JT, Wright EM, Loo DD, Eskandari S. Presteady-state and steady-state kinetics and turnover rate of the mouse gamma-aminobutyric acid transporter (mGAT3). J Membr Biol 190: 57–73, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Sala-Rabanal M, Loo DD, Hirayama BA, Turk E, Wright EM. Molecular interactions between dipeptides, drugs and the human intestinal H+-oligopeptide cotransporter hPEPT1. J Physiol 574: 149–166, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sato K, Adams R, Betz H, Schloss P. Modulation of a recombinant glycine transporter (GLYT1b) by activation of protein kinase C. J Neurochem 65: 1967–1973, 1995 [DOI] [PubMed] [Google Scholar]
- 24. Sun AQ, Ponamgi VM, Boyer JL, Suchy FJ. Membrane trafficking of the human organic anion-transporting polypeptide C (hOATPC). Pharm Res 25: 463–474, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Tiwari S, Riazi S, Ecelbarger CA. Insulin's impact on renal sodium transport and blood pressure in health, obesity, and diabetes. Am J Physiol Renal Physiol 293: F974–F984, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Vallon V, Platt KA, Cunard R, Schroth J, Whaley J, Thomson SC, Koepsell H, Rieg T. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 22: 104–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wright EM, Hirsch JR, Loo DD, Zampighi GA. Regulation of Na+/glucose cotransporters. J Exp Biol 200: 287–293, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733–794, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Wright EM, Loo DD, Hirayama BA, Turk E. Surprising versatility of Na+-glucose cotransporters: SLC5. Physiology 19: 370–376, 2004 [DOI] [PubMed] [Google Scholar]