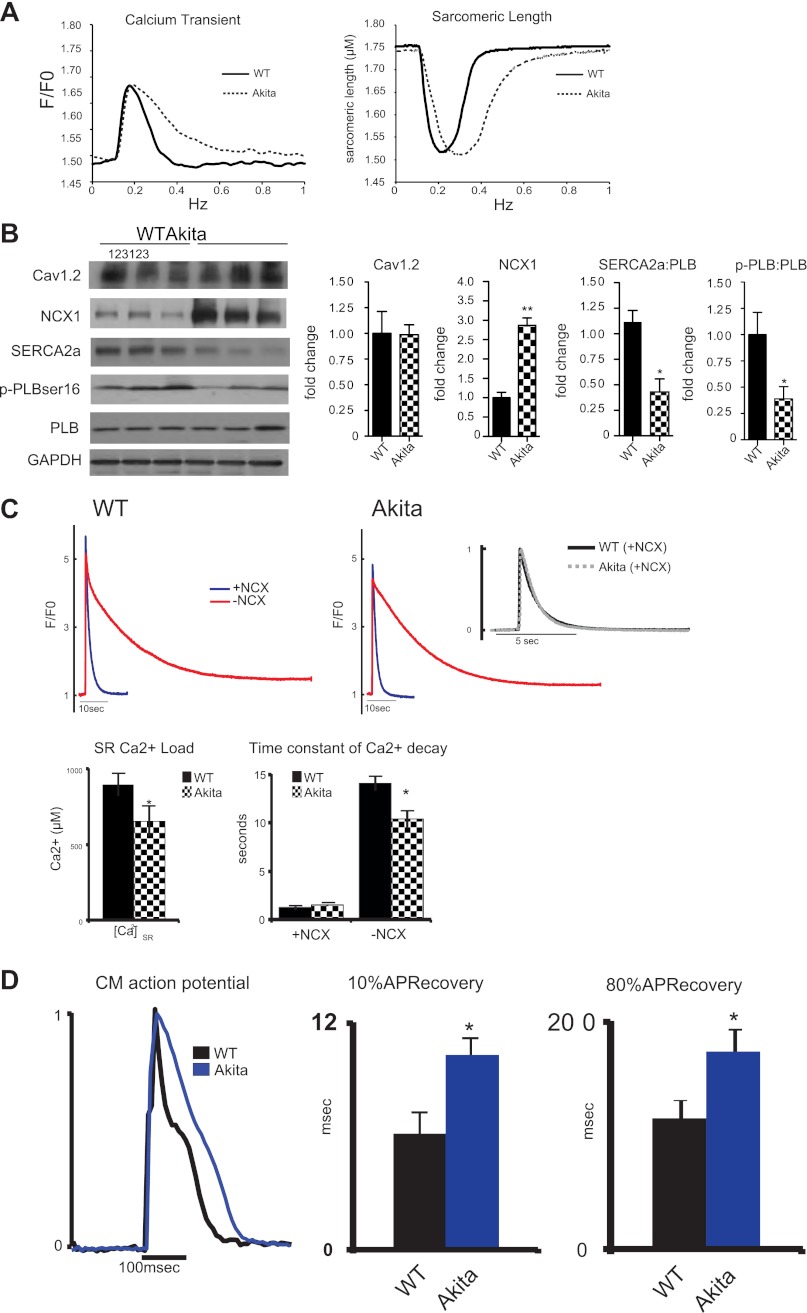
Fig. 3.
Cardiomyocyte mechanics and expression of calcium regulatory proteins. Isolated cardiac myocyte studies with representative whole cell calcium transients and sarcomeric shortening (A). Akitains2 myocytes (dashed) display diastolic dysfunction with increased sarcomeric relaxation and calcium reuptake compared with wild-type (solid); however, peak sarcomeric shortening and peak calcium transient are unchanged between the WT and Akitains2. B: representative Western blot analyses of Cav1.2, Na+/Ca2+ exchanger 1 (NCX1), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2a), and phospholamban (PLB) in the Akitains2 mouse, with quantitative densitometry. pPLB:PLB and SERCA2a:PLB ratios were decreased to <50% of WT levels. Data shown are means ± SD; n = 5 animals/group. *P < 0.05; **P < 0.01. C: caffeine-induced sarcoplasmic reticulum (SR) Ca2+ content in Akitains2 compared with WT. SR Ca2+ and transient decay rates (NCX forward mode function) were both determined by rapid exposure to 20 mM caffeine in 0 Ca2+ and 0 Na+ Tyrode buffer, effectively blocking NCX (−NCX, red tracing) and 0 Ca2+ and 140 mM Na+ to monitor decay rates with functional NCX (+NCX, blue tracing). Time constant of decay (inset) did not show a significant change between the two groups when NCX was active. D: action potential kinetics in cardiomyocytes (CM) from Akitains2 and WT measured using di-8-ANEPPS; 10% and 80% APD recovery in the Akitains2 was significantly longer than WT controls (*P < 0.05) (n = 4 animals/group; >15 cells/animal).