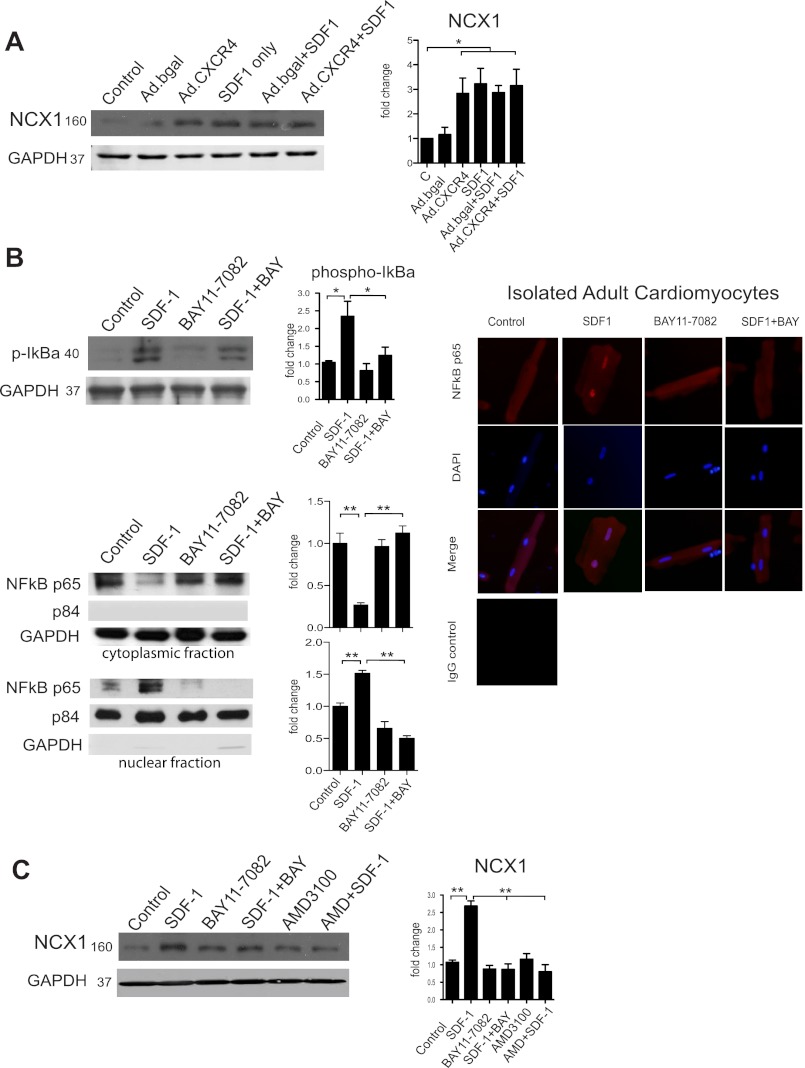
Fig. 6.
CXCR4 regulates NCX1 expression via a NF-κB dependent mechanism. Representative western blots are shown with quantitative densitometry. Adenoviral overexpression of CXCR4 (Ad.CXCR4) in isolated adult rat cardiomyocytes cultured for 24 h produced a significant increase in NCX1 expression compared with Ad.βgal (A). Isolated myocytes were also treated with CXCL12 (SDF1; 100 ng/ml, 24 h) inducing significant expression of NCX1 (A). B: Western blot analysis of CXCL12/SDF1 and BAY11–7082 (5 μM) treatment on IκB-α phosphorylation and NF-κB p65 translocation. Additionally, immunofluorescence staining of NF-κB p65 is shown in similarly treated cardiomyocytes indicating increased p65 nuclear localization with CXCL12/SDF-1. C: isolated myocytes were treated with the IκB-α inhibitor, BAY11–7082 (5 μM) along with SDF1 or AMD3100, a CXCR4 inhibitor, for 24 h. Myocytes treated with BAY11–7082 or AMD3100 prevented CXCL12 induced NCX1 expression indicating a NF-κB-dependent mechanism. Protein loading and purity of fractions were verified by GAPDH (cytoplasmic) and p84 (nuclear). *P < 0.05; **P < 0.01