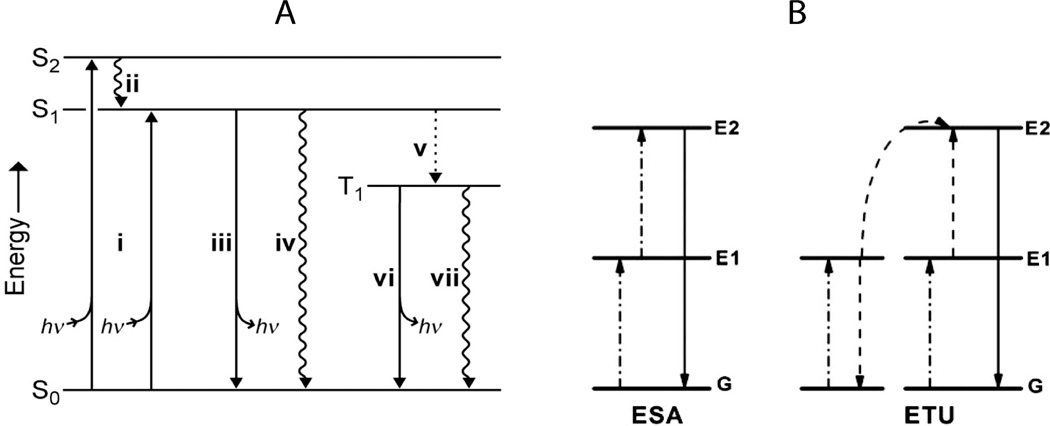
Fig. 3.
Jablonski diagrams showing the main energy pathways for different modes of fluorescence (energy diagrams not drawn to scale). A – single photon Stokes process (i) photon absorption gives excited state, (ii) internal conversion to S1, first singlet excited state, (iii) fluorescence (emission of photon), (iv) nonradiative decay, (v) intersystem crossing to T1 (‘forbidden’ triplet excited state), (vi) phosphorescence, and (vii) nonradiative decay.24 B – two-photon upconversion (anti-Stokes process). Excited-state absorption (ESA) proceeds via sequential absorption of two photons to give the excited state and a subsequent emission event. In energy transfer upconversion (ETU), an ion directly absorbs one photon while a neighboring ion absorbs another and transfers the energy to the first ion, resulting in upconversion and emission.22 Reproduced from Lavis and Raines24 and Haase and Schafer.22