Figure 8.
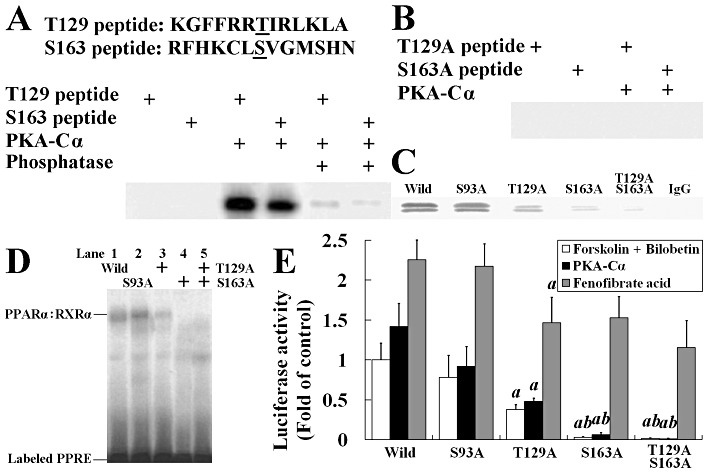
Threonine-129 (T129) and serine-163 (S163) of PPARα are phosphorylated by PKA. (A) Two synthetic peptides containing residues 123 through 135 and 157 through 169 of PPARα were phosphorylated by using 1 U of PKA-Cα in the presence of [γ-32P]-ATP. Some phosphorylated proteins were dephosphorylated by calf alkaline phosphatase to indicate the covalent binding of 32P to threonine or serine. (B) The mutated short peptides with their PKA phosphorylation sites replaced by alanine were incubated with PKA and [γ-32P]-ATP in phosphorylation buffer. (C) Western blot of immunoprecipitated nuclear PPARα from wild or mutated PPARα expression plasmid transfected HEK293 cells. (D) EMSA using extracts from wild-type or mutated PPARα expression plasmid transfected HEK293 cells. The PPRE bindings were observed in wild-type, Ser93A and Thr129A, but not in Ser163A and Thr129A/Ser163A cells. (E) Luciferase activity in HEK293 cells transfected with reporter plasmid (0.5 µg pGL3-SV40-3PPRE with 0.01 µg pRL-CMV as control) and either wild-type or mutated PPARα expression plasmid (0.1 µg) and 0.1 µg pSG5-RXRα plasmids. Cells were treated with forskolin + bilobetin (both 1 µmol·L−1) or fenofibrate acid (1 µmol·L−1) for 4 h. Data are means ± SEM. n= 3. aP < 0.01 versus pSG5-Ser93APPARα transfected cells, bP < 0.01 versus pSG5-Thr129APPARα-transfected cells (anova).
