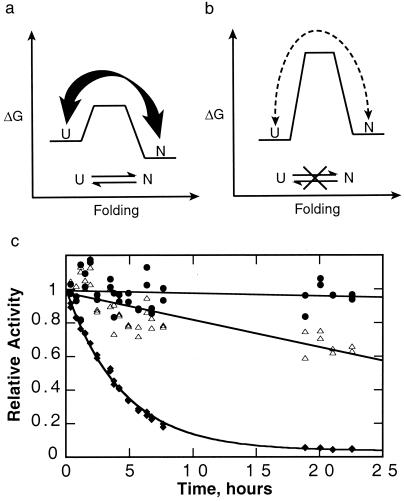
Figure 5.
Advantages of kinetic stability. (a) A typical thermodynamically stable protein without a large barrier samples fully and partially unfolded states, making it susceptible to proteolysis. (b) A kinetically stable protein only rarely samples these unfolded states, making it much more resistant to proteolysis. In the case of αLP, the native state is less stable than the unfolded states; however, kinetic stability does not require a metastable native state. (c) αLP (●) is more resistant to proteolysis than either trypsin (▵) or chymotrypsin (♦). αLP (purified as described in ref. 25), trypsin (TPCK-treated, Worthington), and chymotrypsin (TLCK-treated, Worthington) (6.5 μM each) were mixed in 10 mM CaCl2, 50 mM Mops (pH 7.0) at 37°C. Aliquots were removed over time, and the survival of the individual proteases was measured based on their activities, which could be distinguished given their nonoverlapping specificities for different substrates (succinyl-Ala-Pro-Ala-pNA, succinyl-Ala-Ala-Pro-Arg-pNA, succinyl-Ala-Ala-Pro-Leu-pNA, used for αLP, trypsin, and chymotrypsin, respectively, all at 1 mM in 100 mM Tris, pH 8). Whereas αLP activity decreases at a rate of less than (600 hr)−1, chymotrypsin and trypsin are inactivated with rates of (4 hr)−1 and (60 hr)−1, respectively.