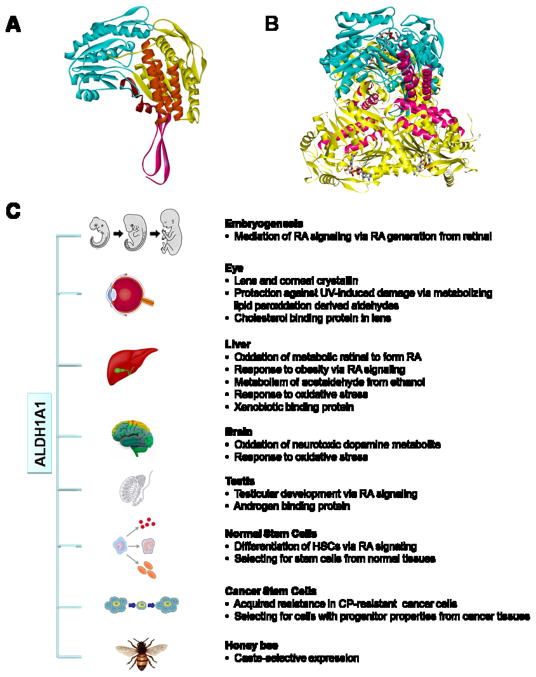
Fig. 1. Protein structure and function of ALDH1A1.
(A) Homology model of human ALDH1A1 monomer showing NAD binding domain (yellow), catalytic domain (blue), oligomerization domain (pink) and three predicated coiled-coil motifs (orange) inside the NAD binding domain. (B) Superimposition of human ALDH1A1 monomer (blue) onto sheep liver ALDH1A1 trimer (yellow), with coiled coil motifs highlighted in pink. Bound NAD for each monomer is shown as stick figure. (C) Physiological and toxicological roles of of ALDH1A1. ALDH1A1 is catalytically active towards the oxidation of wide varieties of aldehyde substrates derived from endogenous or exogenous precursors. In addition, ALDH1A1 is capable of non-catalytic interactions with chemically-diverse compounds. Through its metabolic and non-metabolic properties, ALDH1A1 is involved in multiple physiological and toxicological processes (details are presented in the text). ALDH1A1, aldehyde dehydrogenase 1A1. RA, retinoic acid. DOPAL, 3,4-dihydroxyphenylacetaldehyde. HSCs, hematopoietic stem cells. CP, cyclophosphamide.