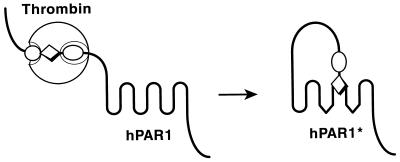
Figure 1.
Mechanism of PAR1 activation. Thrombin (large sphere) recognizes the amino-terminal exodomain of the G protein-coupled thrombin receptor PAR1. This interaction utilizes sites both amino-terminal (P1–P4, small sphere) and carboxyl-terminal (P9′–P14′, small oval) to the thrombin cleavage site. Thrombin cleaves the peptide bond between receptor residues Arg-41 and Ser-42. This serves to unmask a new amino terminus beginning with the sequence SFLLRN (diamond) that functions as a tethered ligand, docking intramolecularly with the body of the receptor to effect transmembrane signaling. hPAR1, human PAR1; the asterisk indicates the activated form. Synthetic SFLLRN peptide will function as an agonist, bypassing the requirement for receptor cleavage.