Abstract
Hibernation is an adaptation to overcome periods of resource limitation often associated with extreme climatic conditions. The hibernation season consists of prolonged bouts of torpor that are interrupted by brief interbout arousals. Physiological mechanisms regulating spontaneous arousals are poorly understood, but may be related to a need for gluconeogenesis or elimination of metabolic wastes. Glutamate is derived from glutamine through the glutamate-glutamine cycle and from glucose via the pyruvate carboxylase pathway when nitrogen balance favors formation of glutamine. The present study tests the hypothesis that activation of NMDA type glutamate receptors (NMDAR) maintains torpor in arctic ground squirrel (AGS; Urocitellus parryii).Administration of NMDAR antagonists MK-801 (5mg/kg,ip) that crosses blood-brain barrier and AP5 (5mg/kg,ip) that does not cross the blood brain barrier induced arousal in AGS. Central administration of MK-801 (0.2, 2, 20 or 200 μg; icv) to hibernating AGS failed to induce arousal. Results suggest that activation of NMDAR at a peripheral or circumventricular site is necessary to maintain prolonged torpor and that a decrease in glutamate at these sites may contribute to spontaneous arousal in AGS.
Keywords: Hibernation, arctic ground squirrel, glutamate, NMDAR, MK-801, circumventricular organ
INTRODUCTION
Hibernation is an adaptation to seasonal periods of resource limitation (Carey et al. 2003, Drew et al. 2007). Hibernation in arctic ground squirrel (Urocitellus parryii) is characterized by periods of profound decreases in core body temperature (Tb) and metabolic rate, termed torpor (Drew et al. 2007, Boyer & Barnes 1999). A torpor bout consists of three phases; entrance, maintenance, and arousal (Boyer & Barnes 1999, Carey et al. 2003, Drew et al. 2007, Heldmaier et al. 2004). Spontaneous, energetically expensive interbout arousals interrupt periods of prolonged torpor throughout the hibernation season (Geiser 1988, Buck & Barnes 2000, Karpovich et al. 2009). Although we and others have shown that torpor onset is mediated via central A1 adenosine receptors (Shintani et al. 2005, Jinka et al. 2011) the mechanisms regulating spontaneous arousals are poorly understood (Harris & Milsom 2000, Drew et al. 2007). Spontaneous arousals may be related to a need for gluconeogenesis (Galster & Morrison 1975) or elimination of metabolic wastes (Drew et al. 2007, Carey et al. 2003). Glutamate, is an amino acid derived from glucose that is also involved in nitrogen balance through the glutamateglutamine cycle (Daikhin & Yudkoff 2000). Glutamate is thus poised to reflect a decrease in metabolic fuel and increase in metabolic waste. Glutamate mediates its effects through NMDA, AMPA, kainate and metabotropic receptors (Siegel & Agranoff 1999). The NR1 subunit of NMDA receptors (NMDAR) are widely distributed throughout the body suggesting that functional NMDAR may reside outside of the CNS (Gill et al. 2000). Given glutamate's role in metabolism and the ubiquitous distribution of NMDAR we hypothesized that a decrease in glutamate might signal arousal from hibernation in AGS via activation of NMDAR. To test this hypothesis, NMDAR antagonists were delivered to torpid AGS and arousal from torpor was measured using respirometry or by behavioral observations.
MATERIALS AND METHODS
Animals
All procedures were in accordance with and approved by the UAF Institutional Animal Care and Use Committee. Arctic ground squirrels (Urocitellus parryii) were captured in the northern foothills of the Brooks Range in Alaska (66°38′N, 149°38′W) and transported to the animal facility at the Institute of Arctic Biology, University of Alaska Fairbanks under permit by the State of Alaska Department of Fish and Game. Animals were maintained on a diet of rodent chow, with daily supplements of carrots and apples, and water ad lib at an ambient temperature (Ta) of 20°C and natural lighting for their wild-trapped latitude. Diet was supplemented with sunflower seeds from August 1 until August 15 when AGS were moved to environmental chambers set to an ambient temperature (Ta) of 2°C and a 4:20-h light-dark cycle. After moving to environmental chambers carrots and apples were discontinued. At the time of testing body weights varied from 634 g to 849 g with a mean±SEM of 764±7 g. All the animals used were males except one female where indicated.
Surgery
Under sterile conditions, telemetry transmitters (model TA-F40 and CTA-F40, Data Sciences International) and an intracerebroventricular (icv) cannula were implanted under isoflurane anesthesia. The transmitter was implanted in the intraperitoneal cavity as described previously (Jinka et al. 2011). The head was leveled in a rat stereotaxic frame (Stoelting). Copalite® (Cooley & Cooley) was applied to the skull. A target was marked at APEBZ +8.5mm, LEBZ + 3.0mm, the arm tilted 15° and the cannula tip repositioned on the target. An internal cannula extending 1.0mm beyond the guide cannula was connected to a syringe primed with sterile saline. The cannula assembly was lowered 5.5mm from the brain surface and retracted until CSF was withdrawn. The guide cannula was secured to anchoring screws (Stoelting) and plugged with a dummy cannula (Plastics One). Animals received enrofloxacin (Bayer Health Care,) (5mg/kg, sc BID for 3 days), and ketoprofen (Fort Dodge Animal Health) (1mg/kg, QD, sc for 3 days total). When CTA-F40 transmitters were used animals received buprenorphine (Hospira) (0.03mg/kg, QD, im for 3 days) and 2 weeks separated transmitter surgery and icv cannula surgery. Following surgery, animals were housed at 20°C 4:20-h L:D and wounds cleaned for at least 10 days before returning to environmental chambers at 2°C. Surgery was performed at least 1 month prior to drug testing.
Rate of O2 consumption and temperature monitoring
A cylindrical Plexiglas metabolic chamber (dia. 28cm, height 23cm) on a rat-turn (Bioanalytical Systems, Inc.) was positioned over a telemetric receiver and Tb was acquired using DataQuest software A.R.T.2.3 (Data Sciences International). Air was drawn from a gas tight swivel at the bottom of the chamber, filtered, passed through a mass flow controller at 3L/min (Model, 840, 0-5L/min, Sierra Instruments Inc.), and a subsample was passed through a multiplexing valve system, dried by a Nafion® drier used in reflux mode (model PD-50T-24-PP, Perma Pure LLC) before passing through the O2 and CO2 analyzers (Model FC-1B and CA-2A, Sable Systems International) (Jinka et al. 2011).
Arousal index
To monitor arousal from behavioral observations a nominal arousal index was developed where 0 was deep torpor indicated by a respiratory rate (RR) of less than 5 breaths per minute (bpm), 1 was a RR of 6-10bpm, 2 was RR greater than 10bpm, 3 was observable shivering, 4 was sporadic body movements, 5 was frequent large body movements and 6 was a fully alert and responsive animal.
Drugs
MK-801(Dizocilpine hydrogen maleate, (5R,10S)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine hydrogen maleate, a non-competitive NMDAR antagonist) and AP5 (2-Amino-5-phosphonopentanoic acid, a competitive NMDAR antagonist) were purchased from Sigma-Aldrich, Inc., St. Lois, MO, USA. MDL-72222 (Tropanyl 3,5-dichlorobenzoate, a 5-HT3 antagonist) was purchased from Tocris Bioscience, Ellisville, MO). MDL-72222 was dissolved in 1% dimethyl sulfoxide (DMSO). MK-801 and AP5 was dissolved in saline. Solutions were sterilized by 0.2μm filtration.
Experiments
Drugs were administered to torpid AGS on the 4th day of a torpor bout or AGS were handled on the 4th day of a torpor bout to induce arousal. Arousal from torpor was quantified using the arousal index scale or from measures of Tb and O2 consumption. Prior to ip administration torpid AGS were habituated to ip injections of saline until they were no longer responsive to ip injections. Once habituated, torpid AGS were administered vehicle (1mL/kg) or drug [MK-801, AP5 or MDL-72222 (5mg/kg, ip)]. Arousal was quantified from the arousal index as described above or from an increase in the rate of O2 consumption and core Tb by an experimenter unaware of treatment.
To determine the effect of icv drug delivery, AGS with chronically implanted icv guide cannula and ip temperature transmitters were habituated for the handling that was necessary to introduce an injection cannula into the chronically implanted guide cannula. On the 3rd day of a torpor bout, a cannula primed with MK-801 or saline was introduced through the guide cannula. Approximately 12 hours after inserting the injection cannula saline, or MK-801, was injected into the lateral ventricle using a syringe pump. MK-801 was delivered in escalating doses of 0.2, 2, 20 or 200 μg in 10μL delivered over 1 min. After a cannula primed with the next dose was inserted animals were returned to the metabolic chamber and O2 consumption was recorded for 30 min. If no increase in O2 consumption was noted, MK-801 was administered. This process was repeated every 2.5 hours. After the last injection, the cannula was left in place for 24 hours while O2 consumption and core Tb were monitored. When AP5 or MDL-72222 was delivered ip, the response to the drug or vehicle injections was monitored by measuring O2 consumption. The drugs were delivered by an observer unaware of the treatment. Handling induced arousal was monitored for comparison with drug-induced arousals. After acquiring at least 30 min of baseline O2 consumption and core Tb records, AGS were subjected to handling sufficient to induce arousal from torpor (n=3) and O2 consumption and core Tb were collected.
Statistical analysis
The frequency of arousal between drug and vehicle treated groups was compared by two-tailed Fisher's exact test where arousal was scored as present or absent. Oxygen consumption was compared between groups using a 2-way ANOVA with repeated measures (SAS, v. 9.1). The level of significance was set at p<0.05. Results are reported as means ± SEM unless otherwise indicated.
RESULTS
Systemic MK-801 induces arousal from torpor
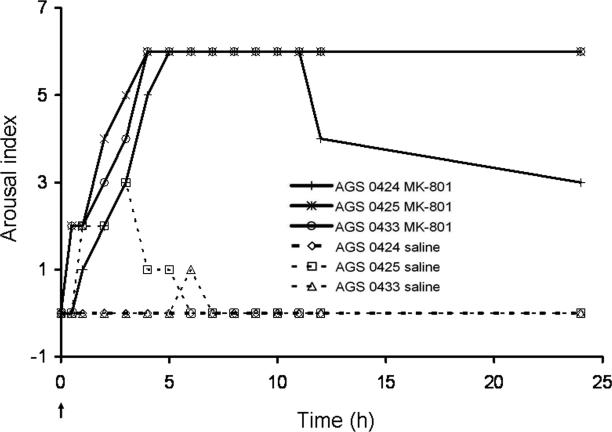
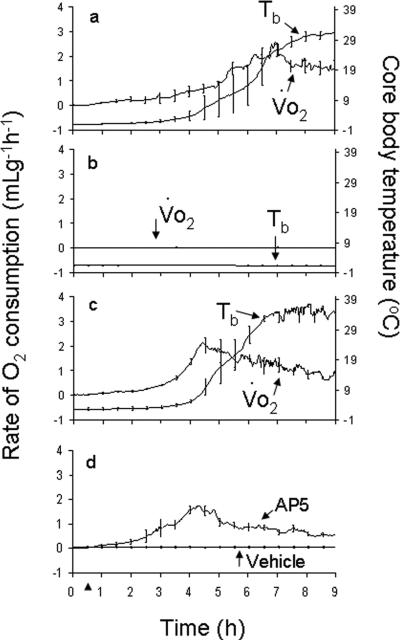
MK-801 (5mg/kg, ip) induced arousal, monitored using the nominal arousal index, in all AGS tested while saline injections did not induce arousal in any of the animals tested (n=3 AGS) (Fig. 1). This result was replicated in a subsequent experiment in which the rate of O2 consumption and core Tb were monitored as indices of arousal. As before, MK-801 (5mg/kg, ip) induced arousal in all of the animals tested (Fig. 2a) while saline injections did not induce arousal in any of the animals tested (Fig. 2b) (n=3). Data shown in Figs. 1 and 2a were combined for Fisher's exact test analysis. The frequency of arousal after MK-801 (5mg/kg, ip) was greater than after saline administration (p<0.01, n=6). To illustrate MK-801-induced arousal relative to handling induced arousal 3 AGS were handled sufficiently to induce arousal and placed into the metabolic chamber (Fig. 2c). MK-801 did not produce any obvious changes in animal behavior and all animals re-entered torpor as expected. The plateau in maximal Tb after MK-801-induced arousal was however lower than has been reported previously for this species during interbout arousal (Karpovich et al. 2009) or after induced arousal (Toien et al. 2001). Lower Tb is likely due to MK-801-induced hypothermia (Corbett et al. 1990).
Fig. 1. NMDAR activation is necessary for maintenance of torpor in AGS.
MK-801 (5mg/kg, ip) or saline was administered to hibernating AGS habituated to ip saline injections. Arousal from hibernation was quantified using an arousal index scale. MK-801, but not saline induced arousal in all AGS tested (n=3 AGS). Arrow represents the time point of injections. Data shown are the arousal index for individual animals. Presence or absence of arousal was combined with data shown in Fig. 2a for statistical analysis; 0424 (♂), 0425(♂), and 0433(♀) are animal identification numbers.
Fig. 2. Arousal induced by inhibiting NMDAR and by handling-induced arousal.
MK-801 (5mg/kg; ip) induced an increase in the rate of O2 consumption (Vo2) and core body temperature (Tb) in all of the animals tested (a) while vehicle had no effect (n=3) (b). Handling-induced arousal (HIA) is shown for comparison (n=3, c). AP5 (5mg/kg; ip) induced arousal from torpor in all of the animals tested while vehicle had no effect in any of the animals tested (n=5) (d). Arrow head at 0.5h represents the time point where either drug (a,d) or vehicle (b,d) was delivered or arousal was initiated by handling (c).
Systemic AP5 induces arousal from torpor
To determine if NMDAR blockade at central or peripheral sites was inducing arousal AP5, an NMDAR antagonist which does not cross blood-brain barrier (Tonkiss & Rawlins 1991), was administered ip. As with MK-801, AP5 (5mg/kg, ip) induced arousal in all of the hibernating AGS tested while vehicle had no effect in any of the animals tested (p<0.01, n=5) (Fig. 2d).
Systemic MDL-72222 does not induce arousal from torpor
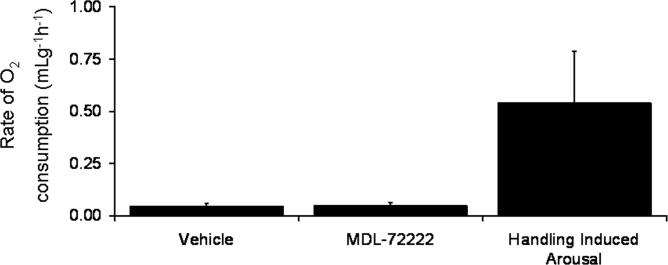
MK-801 has some affinity for 5-HT3 receptors (Yamakura et al. 2000) so to assess the role of 5HT3 receptors in MK-801-induced arousal MDL-7222, a 5HT3 receptor antagonist, was administered ip. Neither MDL-72222 (5mg/kg; ip) nor vehicle induced arousal from torpor (n=3) (Fig 3). The frequency of MDL -72222-induced arousal was significantly less than the frequency of MK-801(5mg/kg, ip)-induced arousal (p<0.02,n=3,6).
Fig. 3. Inhibiting peripheral 5-HT3R does not induce arousal from torpor in hibernating AGS.
Mean rate of O2 consumption measured for 2.5h following MDL-72222 (5mg/kg; ip) or vehicle (n=3) shows that MDL-72222 does not induce arousal from torpor in AGS when compared with HIA.
Intracerebroventricular (icv) administration of MK-801 does not induce arousal
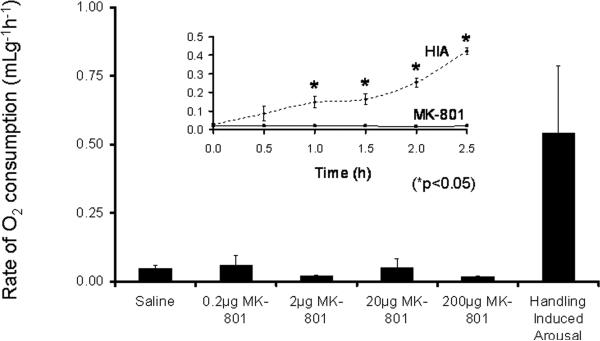
To confirm that MK-801 was acting at a peripheral or circumventricular site MK-801 was administered into the lateral ventricle of AGS habituated to the handling necessary for icv injections. MK-801 was delivered in escalating doses of 0.2, 2, 20 or 200μg (n=3-5), in a volume of 10μL over 1 min. If no increase in O2 consumption was observed within 30min after inserting an injection cannula primed with the next higher dose the drug was administered and O2 consumption monitored for another 2.5h. MK-801 delivered icv did not induce arousal in any of the animals tested (Fig. 4). The metabolic response to handling was greater than the metabolic response to the highest dose of MK-801 (200μg) (n=3;*p<0.05; ANOVA) (Fig. 4 insert).
Fig.4. Administering NMDAR antagonist MK-801 by icv injection does not induce arousal from torpor in hibernating AGS.
Mean rate of O2 consumption for a period of 2.5h following MK-801, icv [0.2μg (n=5), 2.0μg (n=4), 20μg (n=3) or 200μg (n=3) does not differ from mean rate of O2 consumption following saline injection (icv) (n=5)]. Insert shows that handling-induced arousal (HIA) (n=3) produces a significant increase in O2 consumption compared to MK-801 (200μg, icv) (*p<0.05, 2-way ANOVA with repeated measures).
DISCUSSION
Here we show that inhibition of the NMDAR stimulates arousal in AGS. These results are consistent with a previous report that MK-801 (ip) induces arousal in torpid golden mantled ground squirrels (Harris & Milsom 2000). We show further that the site of NMDAR blockade necessary to induce arousal lies outside of the CNS or within circumventricular organs and that the effect of MK-801 is specific to blockade of NMDAR.
The conclusion that the site of MK-801 action lies outside of the CNS, or within circumventricular organs, is supported by the finding that systemic administration of AP5 which does not cross the blood brain barrier (Tonkiss & Rawlins 1991) and MK-801 which crosses the blood-brain barrier (Ozyurt et al. 1988, Park et al. 1988), were equally as effective at inducing arousal. Evidence suggests that the blood brain barrier remains intact during torpor (Wells 1972) so it is unlikely that AP5 reached NMDAR within the CNS except at circumventricular organs where fenestrations in the blood brain barrier allow for communication between the blood and CNS. The interpretation that a CNS site, excluding circumventricular organs, is not involved in MK-801-induced arousal is further supported by failure of direct administration of MK-801 by icv injection at a dose of up to 200μg to induce arousal. Taken together, these results support the conclusion that a central site (excluding circumventricular organs) of NMDAR blockade is not sufficient to induce arousal.
The anatomical location of NMDAR blockade involved in MK-801 and AP5-induced torpor remains to be determined. NMDAR are expressed in high densities within circumventricular organs of AGS, particularly in the median eminence and the area postrema (Zhao et al. 2006). NR1 subunits are also expressed in the kidney, liver, lung, spleen, and testis of rats (Gill et al. 2000) where NMDAR may be important in mediating cardiorespiratory, endocrine and reproductive functions (Gill & Pulido 2001) as well as satiety (Guard et al. 2009). The functional role of NMDAR outside of the CNS remains a matter of debate. All functional NMDAR consist of at least one NR1 subunit in various combinations with two or more NR2A-D subunits. The distribution of NR2 subunits outside of the CNS has not been described, although a limited number of studies have demonstrated functional responses in peripheral tissues (reviewed in Gill et al., 2000).
Similar effects of MK-801 and AP5 also support the interpretation that effects of both drugs are due to NMDAR blockade since there is little overlap in the nonspecific effects of these 2 drugs. MK-801 and AP5 are structurally dissimilar and inhibit NMDAR via distinct mechanisms. MK-801 is a noncompetitive NMDAR antagonist (Collingridge & Singer 1990) that binds to the pore of the NMDAR. By contrast AP5 is a competitive NMDAR antagonist that binds to the NR2 subunit of the NMDAR (Lodge et al. 1988, Watkins et al. 1990, Morley et al. 2005). Specificity of MK-801 was also addressed using a 5HT3 receptor antagonist. In addition to NMDAR, MK-801 inhibits 5-HT3 receptors (Yamakura et al. 2000) leaving the possibility that MK-801 induced arousal via inhibition of 5-HT3 receptors. A role for 5-HT3 receptors is unlikely since MDL-72222 failed to induce arousal. The present study did not, however, address the role of other glutamate receptor families including the other ionotropic receptor families (AMPA and Kainate) or the metabotropic glutamate receptor families.
The function of energetically demanding spontaneous arousals is poorly understood (Harris & Milsom 2000, Drew et al. 2007), but may be related to a need for gluconeogenesis (Galster & Morrison 1975) or elimination of metabolic wastes (Drew et al. 2007, Carey et al. 2003). Glutamate is derived from glucose via the pyruvate carboxylase pathway when nitrogen balance favors formation of glutamine, or from glutamine through the glutamate-glutamine cycle (Hamberger et al. 1979, Oz et al. 2004, Henry et al. 2007, Holten & Gundersen 2008). Blood glucose levels decrease during torpor in hibernating AGS (Osborne et al. 1999) and 13-lined ground squirrel (Andrews et al. 2009). Relative to summer levels, plasma glutamine levels increase during late torpor, remain high during early arousal and begin to decrease during interbout arousal (Epperson et al. 2011). Moreover, in liver, decreases in glucose and increases in glutamine are robust biomarkers of torpor (Serkova et al. 2007). These observations support the hypothesis that glutamate is poised to serve as a neurosignaling molecule that reflects metabolic status. In this way a decrease in NMDAR occupancy could induce arousal from hibernation in response to a need for gluconeogenesis and elimination or recycling of nitrogenous wastes. Further studies are necessary to determine if infusing glutamate will delay arousal, or if infusing a nitrogen donor will induce arousal by shifting the equilibrium towards glutamine.
It is not possible to conclude from the present work if the arousals noted reflect mechanisms involved in spontaneous arousal or mimic an induced arousal. The time course of drug-induced arousal varied and neither the sample size nor the range of doses studied was sufficient to draw conclusions based on comparisons between drug-induced and spontaneous arousal. The time course of drug-induced arousal fell within the range reported in the literature by our lab and others for induced and spontaneous arousal (Toien et al. 2001, Karpovich et al. 2009).
Metabolic pools of glutamate within the cytosol contribute to signal processing (Drew et al. 2004). These same pools participate in extrasynaptic signaling where glutamate is released through cystine/glutamate exchange and reversal of sodium dependent transporters (Kigerl et al. 2012). Extrasynaptic glutamate plays important roles in drug addiction (Moran et al. 2005), homeostatic responses during physiological challenge (Fleming et al. 2011) and excitotoxic response to ischemia (Xu et al. 2009). Alternatively, metabolic control of excitatory neurotransmission also occurs at the synapse. During fasting the ketone body acetoacetate suppresses vesicular glutamate release (Juge et al. 2010). Glutamate is thus poised to convey metabolic needs in the periphery to the nervous system via synaptic and extrasynaptic receptor mediated signal transduction pathways.
In conclusion, our results show that activation of NMDAR at a peripheral or circumventricular site is necessary to maintain prolonged torpor and suggest that a decrease in glutamate may contribute to spontaneous arousal in AGS.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Institutes of Neurological Disorders and Stroke General Medical Sciences of the National Institutes of Health under Award Numbers R15NS070779, NS041069-06 and P20GM103395 and by the US Army Research Office W911NF-05-1-0280 and the US Army Medical Research and Materiel Command 05178001. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the DOD. The authors thank J. Moore and Ø. Tøien and UAF veterinary services for technical support.
Footnotes
Authors declare no conflict of interest.
REFERENCES
- Andrews MT, Russeth KP, Drewes LR, Henry PG. Adaptive mechanisms regulate preferred utilization of ketones in the heart and brain of a hibernating mammal during arousal from torpor. Am J Physiol Regul Integr Comp Physiol. 2009;296:R383–393. doi: 10.1152/ajpregu.90795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer BB, Barnes BM. Molecular and metabolic aspects of hibernation. Bioscience. 1999;49:713–724. [Google Scholar]
- Buck CL, Barnes BM. Effects of ambient temperature on metabolic rate, respiratory quotient, and torpor in an arctic hibernator. Am J Physiol Regul Integr Comp Physiol. 2000;279:R255–262. doi: 10.1152/ajpregu.2000.279.1.R255. [DOI] [PubMed] [Google Scholar]
- Carey HV, Andrews MT, Martin SL. Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol Rev. 2003;83:1153–1181. doi: 10.1152/physrev.00008.2003. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Corbett D, Evans S, Thomas C, Wang D, Jonas RA. MK-801 reduced cerebral ischemic injury by inducing hypothermia. Brain Res. 1990;514:300–304. doi: 10.1016/0006-8993(90)91424-f. [DOI] [PubMed] [Google Scholar]
- Daikhin Y, Yudkoff M. Compartmentation of brain glutamate metabolism in neurons and glia. J Nutr. 2000;130:1026S–1031S. doi: 10.1093/jn/130.4.1026S. [DOI] [PubMed] [Google Scholar]
- Drew KL, Buck CL, Barnes BM, Christian SL, Rasley BT, Harris MB. Central nervous system regulation of mammalian hibernation: implications for metabolic suppression and ischemia tolerance. J Neurochem. 2007;102:1713–1726. doi: 10.1111/j.1471-4159.2007.04675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew KL, Pehek EA, Rasley BT, Ma YL, Green TK. Sampling glutamate and GABA with microdialysis: suggestions on how to get the dialysis membrane closer to the synapse. J Neurosci Methods. 2004;140:127–131. doi: 10.1016/j.jneumeth.2004.04.039. [DOI] [PubMed] [Google Scholar]
- Epperson LE, Karimpour-Fard A, Hunter LE, Martin SL. Metabolic cycles in a circannual hibernator. Physiol Genomics. 2011;43:799–807. doi: 10.1152/physiolgenomics.00028.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TM, Scott V, Naskar K, Joe N, Brown CH, Stern JE. State-dependent changes in astrocyte regulation of extrasynaptic NMDA receptor signalling in neurosecretory neurons. J Physiol. 2011;589:3929–3941. doi: 10.1113/jphysiol.2011.207340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galster W, Morrison PR. Gluconeogenesis in arctic ground squirrels between periods of hibernation. Am J Physiol. 1975;228:325–330. doi: 10.1152/ajplegacy.1975.228.1.325. [DOI] [PubMed] [Google Scholar]
- Geiser F. Reduction of metabolism during hibernation and daily torpor in mammals and birds: Temperature effect or physiological inhibition? J Comp Physiol B. 1988;158:25–37. doi: 10.1007/BF00692726. [DOI] [PubMed] [Google Scholar]
- Gill SS, Mueller RW, McGuire PF, Pulido OM. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicol Pathol. 2000;28:277–284. doi: 10.1177/019262330002800207. [DOI] [PubMed] [Google Scholar]
- Gill SS, Pulido OM. Glutamate receptors in peripheral tissues: current knowledge, future research, and implications for toxicology. Toxicol Pathol. 2001;29:208–223. doi: 10.1080/019262301317052486. [DOI] [PubMed] [Google Scholar]
- Guard DB, Swartz TD, Ritter RC, Burns GA, Covasa M. NMDA NR2 receptors participate in CCK-induced reduction of food intake and hindbrain neuronal activation. Brain Res. 2009;1266:37–44. doi: 10.1016/j.brainres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Hamberger AC, Chiang GH, Nylen ES, Scheff SW, Cotman CW. Glutamate as a CNS transmitter. I. Evaluation of glucose and glutamine as precursors for the synthesis of preferentially released glutamate. Brain Res. 1979;168:513–530. doi: 10.1016/0006-8993(79)90306-8. [DOI] [PubMed] [Google Scholar]
- Harris MB, Milsom WK. Is hibernation facilitated by an inhibition of arousal? . In: Heldmaier G, Klingenspor M, editors. Life in the Cold. Springer-Verlag; Berlin: 2000. pp. 241–250. [Google Scholar]
- Heldmaier G, Ortmann S, Elvert R. Natural hypometabolism during hibernation and daily torpor in mammals. Respir Physiol Neurobiol. 2004;141:317–329. doi: 10.1016/j.resp.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Henry PG, Russeth KP, Tkac I, Drewes LR, Andrews MT, Gruetter R. Brain energy metabolism and neurotransmission at near-freezing temperatures: in vivo (1)H MRS study of a hibernating mammal. J Neurochem. 2007;101:1505–1515. doi: 10.1111/j.1471-4159.2007.04514.x. [DOI] [PubMed] [Google Scholar]
- Holten AT, Gundersen V. Glutamine as a precursor for transmitter glutamate, aspartate and GABA in the cerebellum: a role for phosphate-activated glutaminase. J Neurochem. 2008;104:1032–1042. doi: 10.1111/j.1471-4159.2007.05065.x. [DOI] [PubMed] [Google Scholar]
- Jinka TR, Tøien Ø , Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A1 receptors. J Neurosci. 2011;31:10752–10758. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpovich SA, Tøien Ø, Buck CL, Barnes BM. Energetics of arousal episodes in hibernating arctic ground squirrels. J Comp Physiol B. 2009;179:691–700. doi: 10.1007/s00360-009-0350-8. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Ankeny DP, Garg SK, Wei P, Guan Z, Lai W, McTigue DM, Banerjee R, Popovich PG. System x(c)(-) regulates microglia and macrophage glutamate excitotoxicity in vivo. Experimental neurology. 2012;233:333–341. doi: 10.1016/j.expneurol.2011.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge D, Davies SN, Jones MG, Millar J, Manallack DT, Ornstein PL, Verberne AJ, Young N, Beart PM. A comparison between the in vivo and in vitro activity of five potent and competitive NMDA antagonists. Br J Pharmacol. 1988;95:957–965. doi: 10.1111/j.1476-5381.1988.tb11726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley RM, Tse HW, Feng B, Miller JC, Monaghan DT, Jane DE. Synthesis and pharmacology of N1-substituted piperazine-2,3-dicarboxylic acid derivatives acting as NMDA receptor antagonists. J Med Chem. 2005;48:2627–2637. doi: 10.1021/jm0492498. [DOI] [PubMed] [Google Scholar]
- Osborne PG, Hu Y, Covey DN, Barnes BN, Katz Z, Drew KL. Determination of striatal extracellular gamma-aminobutyric acid in non-hibernating and hibernating arctic ground squirrels using quantitative microdialysis. Brain Res. 1999;839:1–6. doi: 10.1016/s0006-8993(99)01627-3. [DOI] [PubMed] [Google Scholar]
- Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R. Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci. 2004;24:11273–11279. doi: 10.1523/JNEUROSCI.3564-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyurt E, Graham DI, Woodruff GN, McCulloch J. Protective effect of the glutamate antagonist, MK-801 in focal cerebral ischemia in the cat. J Cereb Blood Flow Metab. 1988;8:138–143. doi: 10.1038/jcbfm.1988.18. [DOI] [PubMed] [Google Scholar]
- Park CK, Nehls DG, Graham DI, Teasdale GM, McCulloch J. The glutamate antagonist MK-801 reduces focal ischemic brain damage in the rat. Ann Neurol. 1988;24:543–551. doi: 10.1002/ana.410240411. [DOI] [PubMed] [Google Scholar]
- Serkova NJ, Rose JC, Epperson LE, Carey HV, Martin SL. Quantitative analysis of liver metabolites in three stages of the circannual hibernation cycle in 13-lined ground squirrels by NMR. Physiol Genomics. 2007;31:15–24. doi: 10.1152/physiolgenomics.00028.2007. [DOI] [PubMed] [Google Scholar]
- Shintani M, Tamura Y, Monden M, Shiomi H. Characterization of N(6)-cyclohexyladenosine-induced hypothermia in Syrian hamsters. J Pharmacol Sci. 2005;97:451–454. doi: 10.1254/jphs.sc0040178. [DOI] [PubMed] [Google Scholar]
- Siegel GJ, Agranoff BW. Basic neurochemistry : molecular, cellular, and medical aspects. Lippincott-Raven Publishers; Philadelphia: 1999. [Google Scholar]
- Toien O, Drew KL, Chao ML, Rice ME. Ascorbate dynamics and oxygen consumption during arousal from hibernation in Arctic ground squirrels. Am J Physiol Regul Integr Comp Physiol. 2001;281:R572–583. doi: 10.1152/ajpregu.2001.281.2.R572. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Rawlins JN. The competitive NMDA antagonist AP5, but not the non-competitive antagonist MK801, induces a delay-related impairment in spatial working memory in rats. Exp Brain Res. 1991;85:349–358. doi: 10.1007/BF00229412. [DOI] [PubMed] [Google Scholar]
- Watkins JC, Krogsgaard-Larsen P, Honore T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990;11:25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]
- Wells LA. Permeability of the blood-brain barrier system to rubidium in euthermia, hibernation and hypothermia. Comp Biochem Physiol A Comp Physiol. 1972;42:551–557. doi: 10.1016/0300-9629(72)90133-8. [DOI] [PubMed] [Google Scholar]
- Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330–9343. doi: 10.1523/JNEUROSCI.2212-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Chavez-Noriega LE, Harris RA. Subunit-dependent inhibition of human neuronal nicotinic acetylcholine receptors and other ligand-gated ion channels by dissociative anesthetics ketamine and dizocilpine. Anesthesiology. 2000;92:1144–1153. doi: 10.1097/00000542-200004000-00033. [DOI] [PubMed] [Google Scholar]
- Zhao HW, Christian SL, Castillo MR, Bult-Ito A, Drew KL. Distribution of NMDA receptor subunit NR1 in arctic ground squirrel central nervous system. J Chem Neuroanat. 2006;32:196–207. doi: 10.1016/j.jchemneu.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]