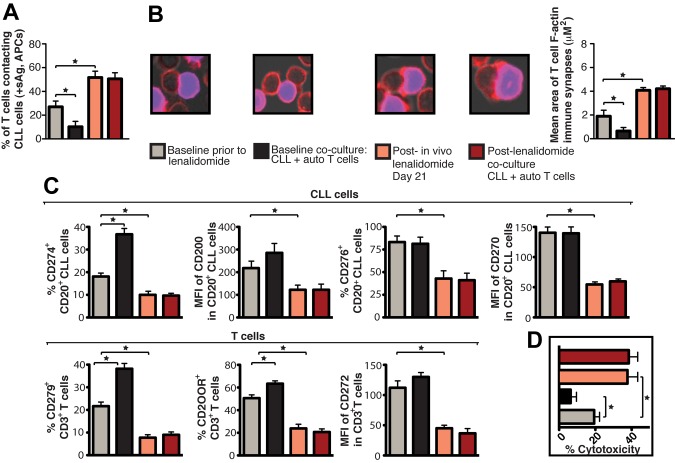
Figure 5.
Lenalidomide in vivo treatment enhances patient T-cell F-actin polymerization at the immunologic synapse with autologous tumor APCs, down-regulates expression of CLL cell inhibitory ligands and T-cell inhibitory receptors, and increases autologous CTL effector function. CD19+ CLL cells and T cells (CD3+ and CD8+) were purified from both pretreatment baseline and after lenalidomide treatment (day 21) samples and used in subsequent analysis. These purified cells were also used in paired ex vivo autologous (auto) coculture experiments (48 hours) with subsequent functional and flow cytometric analysis. (A) Quantification of CD3+ T cells in contact with autologous CLL cells (pulsed with sAg acting as APCs) by immunofluorescence. (B) Mean percent T-cell conjugation ± SD from 3 CLL patients. CD3+ T cell–CLL cell (+sAg) conjugates (n = 100 per experiment) were analyzed for the area (μM2) of F-actin polymerization at the synapse contact site. Mean synapse area ± SD from 3 CLL patients. The confocal images show representative T-cell synapses for each treatment. Original magnification, 63×. (C) Purified CLL cells and CD3+ T cells were examined for inhibitory ligand and receptor expression, respectively, by FACS analysis. Columns show the mean MFI or percent positive expression ± SEM from 3 CLL patients. (D) Mean percent CD8+ T-cell cytotoxicity of autologous CLL cells (+sAg) ± SD from 3 CLL patients (effector-to-target ratio, 30:1) comparing pretreatment baseline and after lenalidomide treatment samples. *P < .05.