Abstract
Background and Aims
Mutualistic ant–plant associations are common in a variety of plant families. Some myrmecophytic plants, such as the epiphytic orchid Caularthron bilamellatum, actively form hollow structures that provide nesting space for ants (myrmecodomatia), despite a substantial loss of water-storage tissue. This study aimed at assessing the ability of the orchid to take up nitrogen from ant-inhabited domatia as possible trade-off for the sacrifice of potential water storage capacity.
Methods
Nitrogen uptake capabilities and uptake kinetics of 15N-labelled compounds (NH4+, urea and l -glutamine) were studied in field-grown Caularthron bilamellatum plants in a tropical moist forest in Panama. Plants were either labelled directly, by injecting substrates into the hollow pseudobulbs or indirectly, by labelling of the associated ants in situ.
Key Results
Caularthron bilamellatum plants were able to take up all tested inorganic and organic nitrogen forms through the inner surface of the pseudobulbs. Uptake of NH4+ and glutamine followed Michaelis–Menten kinetics, but urea uptake was not saturable up to 2 mm. 15N-labelled compounds were rapidly translocated and incorporated into vegetative and reproductive structures. By labelling ants with 15N in situ, we were able to prove that ants transfer N to the plants under field conditions.
Conclusions
Based on 15N labelling experiments we were able to demonstrate, for the first time, that a myrmecophytic orchid is capable of actively acquiring different forms of nitrogen from its domatia and that nutrient flux from ants to plants does indeed occur under natural conditions. This suggests that beyond anti-herbivore protection host plants benefit from ants by taking up nitrogen derived from ant debris.
Keywords: Ant–plant interactions, mutualism, nutrient uptake, Michaelis–Menten kinetics, 15N labelling, myrmecophytes, epiphytes, Caularthron bilamellatum, Orchidaceae, BCNM
INTRODUCTION
Interactions between ants and plants range from very loose associations to obligate and highly specialized mutualisms (Heil and McKey, 2003; Rico-Gray and Oliveira, 2007). Many studies have demonstrated plant protection by opportunistically attracted ants (Oliveira et al., 1999; Sobrinho et al., 2002), though variation in the abundance of ant species or species composition can lead to variation in protective effects (Rico-Gray and Thien, 1989; Di Giusto et al., 2001). In specific and obligate mutualisms, plants offer food rewards and nesting space (specialized hollow structures called ‘myrmecodomatia’), which ensure more constant and long-term associations with ants. In return, the resident ant colony often protects the host-plant against herbivores, fungal pathogens and competing vegetation. The food provided by plants is thought to play an essential role in plant-ant symbioses (Heil and McKey, 2003). It can be provided in liquid form by extrafloral nectaries or glandular trichomes, as energy rich solid food bodies (Fiala and Maschwitz, 1992; Alvarez et al., 2001; Fischer et al., 2002) or may be indirectly acquired from hemipteran trophobionts tended by the ants (Gaume et al., 1998; Stadler and Dixon, 2008). For a long time, flow of resources in such associations was thought to be directed from the plant to its resident ants, but more recent studies have shown that nutrient transfer from ants to plants may also be important as ants accumulate organic matter (e.g. discarded debris or faeces) in their nesting sites that may constitute a nutrient source for their host plant (Treseder et al., 1995; Fischer et al., 2003; Solano and Dejean, 2004). Especially in epiphytes, which often face strong limitation in nutrient availability, the impact of nutrient input by ants on growth and successful reproduction may be substantial (Janzen, 1974; Rico-Gray, 1987; Gay, 1993; Treseder et al., 1995). However, there is a consensus that water is generally even more limiting for growth and survival of epiphytes (Zotz and Hietz, 2001). Thus, the observation that several epiphytic myrmecophytes sacrifice a considerable amount of tissue potentially useful for water storage in order to provide nesting space for ants suggests that ant-provided nutrients are ecologically important. Nutrient transfer has been demonstrated in some well-known myrmecophytic epiphyte genera like Dischidia (Apocynaceae) which form domatia from folded leaves avoiding loss of tissue (Treseder et al., 1995), Lecanopteris (Polypodiaceae), which develop hollow rhizomes (Gay, 1993), or Myrmecodiaand Hydnophytum (Rubiaceae) which exhibit a prominent caudex with natural cavities (Huxley, 1978). Much less studied are two genera of myrmecophytic Orchidaceae which provide hollow pseudobulbs as nesting space for ants: Myrmecophila and Caularthron. Labelling experiments to assess the nutrient uptake capabilities of Myrmecophila (syn. Schomburgkia) tibicinis pseudobulbs were performed by Rico-Gray et al. (1989). Solenopteris ants fed with 14C-labelled glucose were killed and then placed within the pseudobulbs. After 2 weeks of exposure the label could be detected in leaves, roots and pseudobulbs demonstrating carbon uptake from the ant debris. This experiment did not, however, test the uptake of nitrogen or phosphorus, which are most likely severely limiting growth and reproductive success of many epiphytes in situ (Benzing, 1970; Zotz and Hietz, 2001; Zotz and Richter, 2006).
This study focuses on nutrient transfer from ants to plants in Caularthron bilamellatum, a pseudobulb-forming epiphyte distributed from southeast Mexico to Brazil (Govaerts et al., 2010). According to Fisher et al. (1990) the parenchyma tissue inside young pseudobulbs desiccates upon maturation at the onset of the dry season, thus forming a hollow chamber. Ants can enter the hollow pseudobulbs through a vertical slit at the base, which forms during desiccation, and utilize them as nesting space (Dressler, 1981). Thirty-two different ant species were found to be inhabitants of hollow pseudobulbs of Caularthron bilamellatum (Yanoviak et al., 2011). Apart from providing nesting space, the plant attracts ants through extrafloral nectaries on reproductive structures (pedicel, flowers and seedpods), on developing shoots and, as the only known orchid, on mature leaf bases thereby providing nectar throughout the year (Fisher and Zimmerman, 1988). Ants inhabiting the pseudobulbs clearly benefit from this association and, depending on ant species, colony size and alternative food sources, may gain up to half of their nutritional needs from the extrafloral nectaries of their host plants (Fisher et al., 1990). Ant-occupied young pseudobulbs produce significantly more flowers and fruits than those with ants and ant debris removed (Fisher, 1992), but the reason for this has not yet been investigated in detail. Caularthron bilamellatum pseudobulbs lose up to 50 % of their fresh weight (G. Zotz, unpubl. res.) when the hollow chamber develops, which raises the question of a trade-off between water storage capacity and positive effects of the inhabiting ants. We hypothesized that nutrient gain from debris or faeces of the inhabiting ants may constitute such an advantage.
The aim of this study therefore was to demonstrate that Caularthron bilamellatum has the capability to acquire nitrogen through its hollow pseudobulbs and that transfer of this nitrogen to vegetative and reproductive structures occurs. To achieve this, we (a) studied the pseudobulb morphology and inner surface properties to identify possible specialized uptake structures, (b) determined the potential uptake rates and uptake kinetics for ammonium, urea and glutamine, each labelled with the stable isotope 15N, (c) investigated, whether feeding of inhabiting ants with a 15N-labelled bait in the field would lead to 15N uptake into orchid tissue, and (d) monitored a possible translocation of the 15N tracer into reproductive structures of the plant.
MATERIALS AND METHODS
Field study site
The in situ study was conducted from November to December of 2007 in the Barro Colorado Nature Monument (BCNM), Republic of Panama (9°10′N, 79°51′W). The reserve, which consists of various islands such as Barro Colorado Island (BCI) and a number of peninsulas, is almost entirely covered by tropical moist forest receiving an annual precipitation of 2600 mm. The rainy season lasts from April to December, a distinct dry season occurs from late December until March (Croat, 1978; Leigh et al., 1982; Windsor, 1990). Primarily a canopy species, Caularthron bilamellatum is also very abundant on Annona glabra (Annonaceae), a small evergreen tree growing along the southern shoreline of BCI and rarely exceeding 7 m (Croat, 1978; Zotz et al, 1999). Here, this orchid is readily reachable by boat and can easily be sampled and monitored in large numbers.
Sample collection, light microscopy and SEM investigation
For studies of pseudobulb anatomy and surface characteristics, mature, hollow pseudobulbs of different size inhabited by or free of ants, as well as immature pseudobulbs that had not yet formed a hollow chamber, were harvested along the south coast of BCI and fixed in 70 % ethanol for further analyses at the University of Vienna. For light microscopy (LM), samples were embedded in Technovit 7100, a HEMA-based resin (Heraeus Kulzer GmbH, Wehrheim/Ts, Germany). Resin blocks were cut to slices of 5–10 μm using a Leitz 1515 microtome (Leica Microsystems AG, 35578 Wetzlarand, Germany). Samples were either stained with Toluidine Blue to enhance overall contrast, or with Sudan III to test for suberin and cutin in the cell walls. For scanning electron microscopy (SEM), samples were re-fixed in gluteraldehyde overnight, critical point dried in liquid CO2, sputtered with gold and analysed in a JEOL JSM-6390 scanning electron microscope (JEOL USA Inc., Peabody MA, USA).
Labelling experiments
To estimate tissue loss during formation of the pseudobulb chamber, cross-sections of fresh mature pseudobulbs were taken, comparing the overall diameter to the diameter of the pseudobulb cavity. The ratio between total pseudobulb volume and the hollow chamber is expressed by the ratio between the total radius r and the radius of the chamber rc.
To determine the plants potential for nutrient uptake through the pseudobulb chamber, label was carefully injected through the basal slit of mature uninhabited pseudobulbs using a syringe and bulbs were placed upside down for an incubation time of 1 h. We used 15N-labelled NH4Cl (99 at%), urea (98 at%) and l -glutamine (alpha-15N, 98 at%) (Cambridge Isotope Laboratories, Andover, MA, USA) at concentrations of 50, 100, 250 and 500 μm, and 1·0 and 2·0 mm, with three replicates for each concentration and nitrogen form. After incubation, apoplastically bound ions were removed by flushing the hollow pseudobulbs twice with a 10 mm CaCl2 solution and washing the inner and outer surface of the pseudobulbs with distilled water. Small samples of each pseudobulb's apical region were cut out and dried at 50 °C for 48 h. Differences in nitrogen uptake rates between small and large pseudobulbs were not found to be significantly different (two-way ANOVA, P > 0.050). Samples of the two size groups were therefore pooled.
To detect possible translocation of label to reproductive structures, Caularthron bilamellatum plants were collected at Barro Colorado Island and cultivated at the Botanical Garden of Vienna (HBV). At the onset of flower buds, 2 mL of a 2·0 mm 15NH4Cl solution were injected into the pseudobulb cavity, and plants mounted upside down to keep the label in the pseudobulb apex. After 12 weeks the ripened seedpods were harvested and seeds dried at 50 °C for 48 h.
To investigate nutrient transfer from ants to plants isolated trees of Annona glabra along the south coast of BCI and north coast of neighbouring Gigante peninsula carrying Caularthron bilamellatum plants of different sizes and inhabited by different ant species were randomly selected for an ant feeding experiment. A small plastic bottle containing a solution of honey amended with 15NH4Cl was mounted to each host tree (Fig. 1A). Small holes drilled in the upper part of the bottle allowed ants to access the bait while preventing it from leaking or being washed out by heavy rain. The bottle was located beneath the orchids and active roots were removed to prevent contamination by patrolling ants carrying the label. The bait was usually taken up overnight and refilled every 2–3 d. After 2 weeks small plants were harvested in total, while only individual pseudobulbs were sampled from very large plants. Adult ants, larvae, detritus as well as ant carton made by some species were collected from each sampled plant and dried at 50 °C for 48 h. Pseudobulbs were washed, cut, and dried as described above.
Fig. 1.
Morphology of Caularthron bilamellatum pseudobulbs. All material is from plants naturally growing on Annona glabra (Annonaceae) in BCNM, Panama. (A) A small plastic bottle (blue, arrow) containing 15N enriched honey solution was mounted beneath the orchids to determine a possible nutrient transfer from ants to plants. (B) Longitudinal section of an immature pseudobulb showing the transparent parenchyma tissue in the centre and the beginning desiccation at the base as light brown tissue. (C) Cross-section near the apex of a mature hollow pseudobulb not inhabited by ants. (D) Longitudinal section of the apical region of a mature pseudobulb inhabited by a large number of ants. The entire surface is covered with organic material containing remains of prey, dead ants, mites and coccids. (E) Longitudinal section of a mature pseudobulb inhabited by a large number of ants. The entrance is located at the base (right), the surface of the cavity is smooth in the lower third becoming increasingly rougher towards the apex (left) where waste is stored. Ant carton can be seen in the middle regions of the pseudobulb.
Stable isotope analysis
Samples were dried for 24 h at 60 °C and homogenized with a ball mill (RetschMM2, Haan, Germany). Aliquots of 1·5–2 mg were weighed into tin-capsules and subjected to isotope ratio mass spectrometry. For measuring stable nitrogen isotope ratios (15N/14N), an elemental analyzer (EA1110, CE Instruments, Milan, Italy) connected to an isotope ratio mass spectrometer (DeltaPLUS, Finnigan MAT, Bremen, Germany) by a ConFlo II interface (Finnigan MAT) was used. Reference gas (high purity N2, Air Liquide, Vienna, Austria) was calibrated to the atmospheric N2 (at-air) standard using reference material obtained from the International Atomic Energy Agency (Vienna, Austria).
15N incorporation was determined from N concentrations (CN) in dry mass (Md) and the corresponding atom % 15N and at% 15N excess (APE) values.
at% 15N [%] = mol 15N/(mol 15N + mol 14N)
Uptake rates (J) were calculated as follows:
Mr is the molecular weight of 15N and t the incubation time in hours.
Kinetic constants were determined using SigmaPlot11 (Systat Software GmbH, Ekrath, Germany), fitting the uptake values to the Michaelis–Menten equation (regression analysis by hyperbola, single rectangular, two parameters). The equation for the hyberbolic regression was used to determine the Michaelis–Menten constant according to the equation
in which v is the uptake rate at a given substrate concentration [S], Vmax the maximum uptake rate at substrate saturation and Km the Michaelis–Menten constant (Leskovac, 2003; Wanek and Pörtl, 2008). Km and Vmax can also be derived from regression models using Lineweaver–Burk, Eadie–Hofstee and Hanes–Wolf equations (Markus et al., 1976), but as hyperbolic regression delivered the best fitting to the datapoints (R2 > 0.9) as well as the most robust results, it was chosen for further analyses.
Statistical analysis
Statistics were performed using SigmaPlot 12 (Systat Software GmbH, Ekrath, Germany), and STATISTICA 8·0 (StatSoft, Inc. 2008, data analysis software system). Differences between types and concentrations of labelling compounds, as well as between labelled and unlabelled control samples were determined by t-test or analysis of variance (ANOVA), followed by a Holm–Sidak post hoc test where appropriate. Log-transformation was applied to datasets failing to show normal distribution in order to fulfil the criteria for statistical testing. Unless stated otherwise the standard error of the mean (s.e.) was chosen as a measure of variability in all figures and tables.
RESULTS
Pseudobulb anatomy and surface characteristics
Growing pseudobulbs of Caularthron bilamellatum were bright green, fleshy and exhibited a very high water content (Fig. 1B). The centre was completely clear, gel-like and lacked cellular structures (Supplementary Data Fig. S1A). As material was collected at the end of the rainy season, the preformed basal slits had just opened and the desiccation of the parenchyma tissue in the pseudobulbs was at a very early stage. Ants rarely inhabited the small space within these immature pseudobulbs.
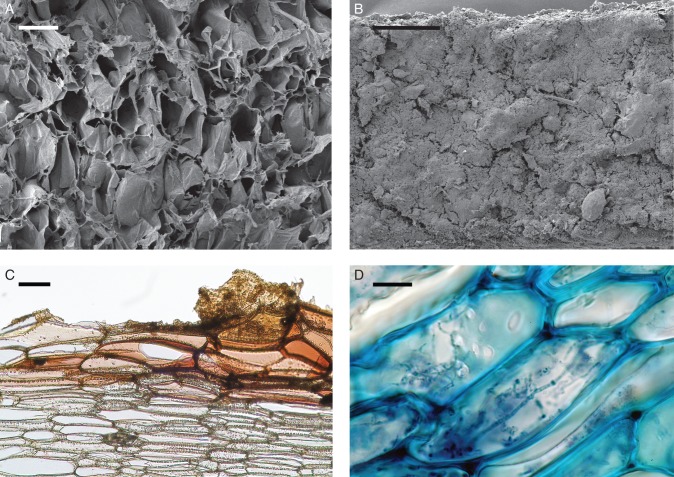
In mature pseudobulbs which had formed during the previous growing season, the parenchyma tissue at the centre had fully desiccated forming a hollow chamber. Those pseudobulbs which were not (Fig. 1C), or only weakly inhabited by ants (Supplementary Data Fig. S1D), exhibited a smooth and yellow brownish inner surface around the slit, turning darker toward the middle region of the pseudobulb, and becoming very rough towards the apex (Supplementary Data Fig. S1D). SEM images revealed a distinct outer layer of unusually large dead and partially torn cells, forming a crater-like surface at the apical regions (Fig. 2A; Supplementary Data Fig. S2B). The cell walls of this outer layer are covered with cutin and suberin accrustations, and the cell walls of the adjacent layers of living cells are densely pitted (Fig. 2C). The surface is highly hydrophilic and shows sponge-like properties; when wetted, moisture is quickly absorbed and distributed across the cavity. Pseudobulbs of all sizes and plant ages shared these features. The colonising ants seem to excavate the remains of the desiccated parenchyma and appear to be responsible for these surface characteristics indicating that at least at some point ants had visited these bulbs.
Fig. 2.
Pseudobulb anatomy of Caularthron bilamellatum, collected in BCNM, Panama. (A) Scanning electron microscope image of a mature, uninhabited pseudobulb cavity near the apex. The rough surface consists of large dead and often torn cells. (A higher magnification of this image can be found in Supplementary Data Fig. S2B). (B) SEM image of a mature ant-inhabited pseudobulb cavity near the apex. The surface is completely covered with a layer of organic material. Note the mite down to the right. (A higher magnification of this image can be found in Supplementary Data Fig. S2D). (C) Light microscopic image of a mature pseudobulb inhabited with only few ants. The cross-section was made near the apex of fresh plant material. Sudan III staining reveals thick suberin/cutin accrustations on the cell walls of the cavity surface. Organic material and few fungal hyphae can be seen in the upper right corner. The cell wall in deeper tissue layers (colourless) are densely pitted. (D) LM image of a mature pseudobulb inhabited by a large number of ants (cross section near the apex). Toluidine Blue staining was used to enhance contrast. Intracellular fungal hyphae are present within the cells below the surface layer. Scale bars: (A) = 200 µm; (B) = 500 µm; (C) = 100 µm; (D) = 25 µm.
In contrast, the interior of mature pseudobulbs inhabited by large numbers of ants at the time of sampling differed in characteristics and colour. Apparently depending on ant species and colony size, the surface was often covered with ant waste and ant-made carton (Supplementary Data Fig. S1C).
Ant waste was preferably stored in the cavity's tip and developed into a dark brownish coat totally covering the cells of the surface (Fig. 1D, E). Remains of prey, dead nest mates, plant material, mites, nematodes and even coccids could be identified in the organic material, although for the largest part it appeared to consist of fungal hyphae (Fig. 2B and Supplementary Data Fig S2C, D). Hyphae were not restricted to the surface but could also be detected in the adjacent layer of living cells (Fig. 2D). Ants of the genera Azteca and Crematogaster were the most common inhabitants of the studied pseudobulbs, primarily Azteca cf velox and Crematogaster crinosa. Azteca cf velox built carton to divide the plant cavity into compartments (Supplementary Data Fig. S1C).
Very few of the examined mature pseudobulbs had failed to form a slit at the base and thereby remained closed. Though the centre had desiccated, the hollow chamber remained completely inaccessible to ants. The interior of such pseudobulbs differed from those exposed to the environment. Remains of desiccated parenchyma cells covered the entire surface of the pseudobulb cavity, giving it a white-yellowish colour (Supplementary Data Fig. S1B).
On average, the hollow chamber took up about 42 % of total pseudobulb volume (range 33 % –53 %, n = 15).
Potential N uptake and kinetics
Pseudobulbs of C. bilamellatum were able to take up all supplied forms of nitrogen and showed significant enrichment in 15N compared with unlabelled controls at all concentrations. Plants preferably took up NH4+, which was taken up significantly faster than urea and glutamine (two-way ANOVA, F8;69= 2·466, Holm–Sidak, both P < 0.001). Uptake rates of urea and glutamine were not significantly different (Holm–Sidak, P > 0.05) from each other (Table 1).
Table 1.
Two-way ANOVA and Holm–Sidak post-hoc test results for the net nitrogen uptake into pseudobulbs of Caularthron bilamellatum collected in BCNM, Panama
| Effect | SS | d.f. | MS | F | P |
|---|---|---|---|---|---|
| Intercept | 3.925153 | 1 | 3.925153 | 74.61640 | <0.000001 |
| Label | 1.446752 | 2 | 0.723376 | 13.75124 | 0.000009 |
| Conc | 2.377757 | 4 | 0.594439 | 11.30018 | <0.000001 |
| Label × conc | 1.037714 | 8 | 0.129714 | 2.46584 | 0.020633 |
| Error | 3.629705 | 69 | 0.052604 |
Three nutrient sources (NH4+, urea or glutamine) labelled with 15N were injected into the plants' hollow pseudobulbs. SS, Single squares; d.f., degrees of freedom; MS, mean squares.
Ammonium and glutamine exhibited Michaelis–Menten type uptake kinetics. In contrast, uptake of urea was linearly related to substrate concentrations within the tested range (up to 2 mm) (Supplementary Data Fig. S3). Calculated Vmax values were 1·01 ± 0·21 µmol 15N g−1 Md h−1 for NH4+ and 0·66 ± 0·07 µmol 15N g−1 Md h−1for glutamine. The affinity of the uptake system was slightly higher for ammonium, with a Km value of 0·41 mM compared to 1 mm for glutamine. Catalytic uptake efficiency, calculated as Vmax/Km, was surprisingly low in both cases, but approx. 3-fold higher for NH4+ than for glutamine (Table 2).
Table 2.
Determination of kinetic constants by non-linear regression analysis of net nitrogen uptake in Caularthron bilamellatum pseudobulb cavities
| Label | Km (μmol) | Vmax (μmol 15N g−1 Md h−1) | R2 | Vmax/Km | P |
|---|---|---|---|---|---|
| Ammonium | 410.94 ± 228.87 | 1.1 ±0.21 | 0.940 | 0.0025 | 0.0190 |
| Glutamine | 998.67 ± 226.14 | 0.66 ±0.07 | 0.988 | 0.0007 | 0.0107 |
| Urea | – | – | 0.999 | – | 0.0006 |
Shown are net nitrogen uptake rates within the domatia of plant material collected in BCNM, Panama. Michaelis–Menten constants (Km) and maximum uptake rates (Vmax) were derived from hyperbolic Michaelis–Menten fit (n = 6). The ratios Km/Vmaxrepresent the catalytic uptake efficiencies and regression coefficients (R2) show the quality of the regression fitting. Urea did not show Michaelis–Menten kinetics (see Supplementary Data Fig. S2).
Translocation of tracer to reproductive structures
Seeds harvested from plants labelled by injecting 15NH4+ solution into the pseudobulb cavity, allowing incubation until capsules matured, were significantly enriched in 15N (t-test, t = –17.311, P < 0.001, ncontrol = 6, nlabelled= 4) exhibiting mean δ15N values of 317.6 ± 23.1 ‰ compared with 1.6 ± 0.25 ‰ of the unlabelled control group (Fig. 3).
Fig. 3.

Translocation of nitrogen taken up by Caularthron bilamellatum pseudobulbs to reproductive structures. Plants collected at BCNM, Panama and cultivated at HBV Vienna, Austria were labelled by injecting 2·0 mm NH4+into the hollow pseudobulbs at the onset of flowering. Seeds were harvested after 12 weeks and compared to an unlabelled control group. Groups were significantly different (t-test, t = –17.311, P < 0.001, ncontrol= 6, nlabelled= 4). Note the logarithmic scale of the y-axis. Error bars represent s.e.
Transfer of label from ants to plants
Ants, which were fed a honey-solution labelled with NH4+, exhibited δ15N values ranging from 148 ‰ to 1457 ‰. In contrast, larval stages, which were only present in sufficient numbers for mass spectrometry in two samples, showed a low enrichment with δ15N values (10·2 and 12·4 ‰). Ant carton yielded intermediate δ15N values (69·8 ± 36·0 ‰), the relative amount of label present in ant carton and plants varied greatly between the different sampling sites (Supplementary Data Table S1).
Samples taken from the apical pseudobulb regions of plants inhabited by labelled ants were also significantly enriched in 15N (t-test, t = 5.600, P < 0.001, ncontrol= 8, nlabelled= 90) exhibiting mean δ15N values of 165.4 ± 34·5 ‰ compared with the control group with 0·61 ± 0·58 ‰ (Fig. 3). However, the amount of nitrogen taken up varied substantially between each plant and also in neighbouring pseudobulbs of the same plant, with δ15N values ranging between 5·1 ‰ and 1684 ‰, presumably due to differences in ant visits. Generally the amount of incorporated 15N decreased from apex (106.5 ± 17·6 ‰) over centre (79.6 ± 16.0 ‰) to base of the pseudobulbs (50.8 ± 8.3 ‰) where the slit is located (one way ANOVA, F2;24 = 3.690, P < 0.05). The difference between apex and base was significant (Holm–Sidak, P < 0.05) but not between apex and centre or centre and base (Holm–Sidak, P > 0.05), (Fig. 4; Supplementary Date Table S2).
Fig. 4.
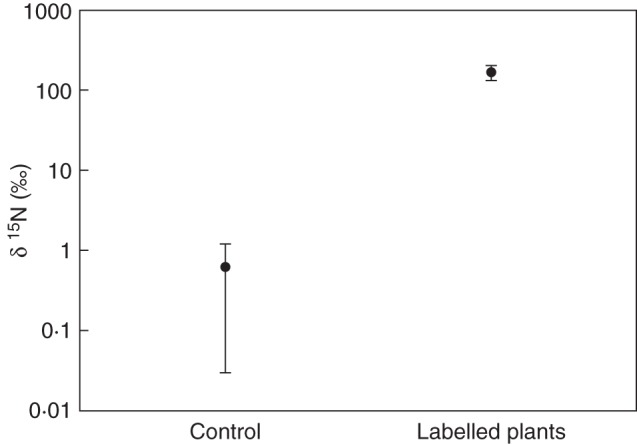
Nitrogen transfer from ants inhabiting pseudobulbs into plant tissue. Ants inhabiting specimens of Caularthron bilamellatumgrowing naturally on Annona glabra at BCNM, Panama, were labelled by feeding them a solution of honey containing 15NH4Cl. Ants transported the label into the hollow pseudobulbs. These were harvested after 2 weeks and compared with an unlabelled control group. Groups were significantly different (t-test, t = 5.600, P < 0.001, ncontrol= 8, nlabelled= 90). Note the logarithmic scale of the y-axis. Error bars represent the s.e.
Fig. 5.
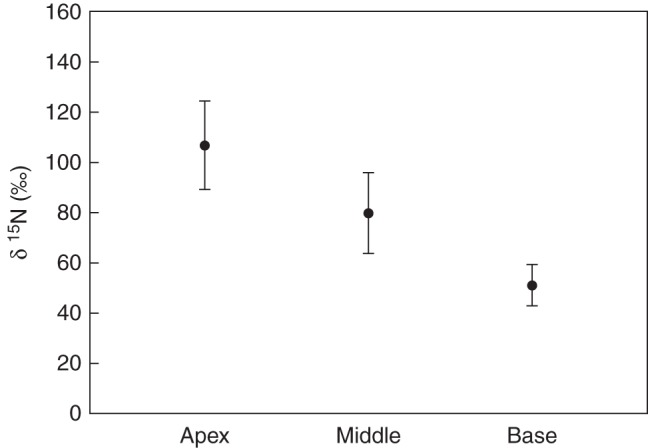
Spatial variations of 15N uptake between basal, middle and apical regions of mature pseudobulbs of Caularthron bilamellatum. For details compare legend of Fig. 3. Spatial distribution of label within pseudobulbs was significantly different between apical and basal regions (one way ANOVA, F2;24 = 3.690, Holm–Sidak, P < 0.05) but not between apical and middle or middle and basal regions (Holm–Sidak, P > 0.05) (see Supplementary Data Table S2). Error bars represent s.e. (n = 9).
Immature pseudobulbs that were still mostly filled with parenchyma tissue exhibited a highly significant (t-test, t = –16.251, P < 0.001, nbase = 3, nmiddle = 3) opposite trend: a higher amount of label was present at the desiccated base (84.61 ± 6.35 ‰), which was accessible for ants, but declined towards the middle sections (20.51 ± 0.95 ‰) still filled with parenchyma tissue and therefore inaccessible to potential inhabitants. (Supplementary Data Fig. S4).
DISCUSSION
Most vascular epiphytes face limited and/or irregular supply of nutrients, demanding highly specialized adaptations, such as myrmecophily (Benzing, 1970). Even though research of ant–plant interactions in myrmecophytes has long focused on nutrient transfer from the plant to inhabiting ants, which in return defend their host against herbivores, encroaching vegetation and fungal pathogens (Rico-Gray and Oliveira, 2007), there is increasing evidence that nutrient acquisition has to be recognized as another direct benefit to the host-plant, especially in the case of myrmecophytic epiphytes. It has already been demonstrated that a number of myrmecophytic epiphytes in different plant families are capable of utilizing nutrients provided by inhabiting ants in form of waste and faeces (Janzen, 1974; Rico-Gray, 1987; Gay, 1993; Treseder et al., 1995), but detailed information is still scarce.
Based on 15N labelling experiments, our results for the first time provide direct evidence that transfer of nitrogen from ants to plants indeed occurs in a myrmecophytic epiphytic orchid. By labelling associated ants it was demonstrated unequivocally that Caularthron bilamellatum has the potential to take up nitrogen from ant waste through its hollow pseudobulbs under field conditions. The spatial distribution of labelled compounds within pseudobulbs, i.e. a stronger label in apical parts of mature bulbs, may have two reasons. First, inhabiting ants generally tend to store their waste in the apical part of the pseudobulb and keep the entrance at the base clean, which leads to a concentration of detritus in the apical part. Second, the roughness of pseudobulb surfaces strongly increases towards the apex due to large and partly torn cells present in this area. Although not comparable to highly specialized surface structures like warts in myrmecophytic Rubiaceae (Huxley, 1978; Rickson, 1979) these structures increase the total surface considerably and therefore provide a higher waste storage capability. The strongly pitted cell walls in both, the outer layer of dead cells and in the adjacent layers of living cells below, may help to maintain nutrient permeability despite wall accrustation with suberin and cutin in the outermost surface cells. Though the surface has some sponge-like properties, a similarity to the velamen radicum of roots is not suggested. The cavity surface consists only of a single to very few layers of large dead cells with thick walls and is lacking typical helical cell wall thickenings of a velamen radicum. (Benzing et al., 1982).
The growth of many epiphytes is thought to be limited by nutrients such as nitrogen or phosphorus (Laube and Zotz, 2003; Winkler and Zotz, 2008). It is therefore tempting to speculate that the input of nitrogen from ants established in this study may be beneficial for the growth of Caularthron bilamellatum. In additional experiments we were also able to demonstrate nitrogen transfer into seeds, indicating that nutrient input into pseudobulbs may also be beneficial for the plants' reproduction.
Nitrogen uptake kinetics for different organic and inorganic nitrogen sources showed significant and active uptake of all offered nitrogen forms. It is well known, for example for NH4+, that plant roots often exhibit uptake kinetics dominated by high affinity transport systems (HATS) at substrate concentrations up to 1 mm, consisting of highly sensitive but quickly saturable transport proteins usually expressed under nutrient starvation (von Wiren et al., 2000). Above about 1 mm low affinity transport systems (LATS) with low substrate affinity, but high uptake capacity take over, facilitating uptake at larger substrate concentrations. For Caularthron bilamellatum we calculated a Km value of about 0·4 mm for NH4+ at a relatively low Vmax of about 1 µmol 15N g−1 Md h−1, representing small catalytic uptake efficiency. In studies with soft-bodied plants such as macroalgae and bryophytes (lacking a distinct cuticula) Km values in the range of 0·5–500 µm were found for ammonium and amino acid transport systems in leaves, but at a higher Vmax causing distinctively higher catalytic uptake efficiencies than in this study (Tyler et al., 2005; Wanek and Pörtl, 2008). However, Vmax and Km values comparable to those found for the inner surface of the pseudobulbs in our experiments have been reported for amino acid and ammonium uptake by leaf tissue of an epiphytic tank bromeliad (Inselbacher et al., 2007). Interestingly, in both cases a linear uptake of urea up to a concentration of several mm was found, indicating low-affinity uptake systems, as a possible adaption to exploit the infrequent but intense nitrogen input by animal excretions. Such versatile uptake capacities seem especially important for epiphytes adapted to nutrient-poor ecosystems, which have to deal with a broad variety of scarce or only temporarily available forms of nitrogen (Lambers et al., 1998) demanding a high flexibility to acquire potential nutrient sources.
Myrmecophytic epiphytes like Caularthron bilamellatum share some similarities with carnivorous plants, which have also developed sophisticated strategies to survive in extremely nutrient-poor environments (Juniper et al., 1989, Krol et al., 2012). The traps of some carnivorous plants, similar to ant domatia, often resemble microenvironments containing a large number of different organisms which may help to degrade detritus thereby increasing nutrient uptake of the host plant (Blatrix et al., 2009, Paracer and Ahmadjian, 2000). In comparison with true carnivorous plants in a strict sense (Givnish et al., 1984), active prey caption or glands secreting digestive enzymes are missing in Caularthron bilamellatum. Some protocarnivorous plants, such as Roridula gorgonias, however, are also missing glands and digestive enzymes and do not digest trapped insects directly. They rather use the faeces of a mutualistic bug from the genus Pameridea Reut. (Heteroptera, Miridae) which feeds on trapped insects (Juniper et al., 1989), thereby acquiring significant amounts of nitrogen (Ellis and Midgley, 1996; Anderson, 2005). Even though the amount and importance of nutrient transfer from symbiont to plant has yet to be quantified in many systems, these results suggest that plants have many and often subtle ways of exploiting animals as food sources.
In summary, we were able to demonstrate that Caularthron bilamellatum plants are capable of taking up nutrients (a) from organic matter deposited by ants at the inner surface of the hollow pseudobulbs and (b) from different organic and inorganic nitrogen forms injected into the pseudobulb cavity in liquid form. Uptake kinetics of the inner surface of the hollow pseudobulbs were comparable to results obtained from leaves of epiphytic bromeliads suggesting the presence of active transport systems capable of dealing with a broad variety of compounds and concentration ranges. As nitrogen was also translocated into reproductive structures we speculate that nutrient input by ants may generally increase plant fitness (vegetative growth and reproduction). All these features are especially useful for an epiphytic myrmecophyte having to cope with a harsh, unpredictable and nutrient-poor habitat where associations with ants acting both as a potential protection and a constant supply of nutrients may be the key to survival.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Margarete Watzka for help with stable isotope analyses, Susanne Sontag for help and advice in anatomical studies and electron microscopy. The Panamanian authorities (ANAM) granted export permits (SEX/P-01-10 and SEX/AP-05-07).
LITERATURE CITED
- Alvarez G, Armbrecht I, Jimenez E, Armbrecht H, Ulloa-Chacon P. Ant–plant association in two Tococa species from a primary rain forest of Colombian Choco (Hymenoptera: Formicinae) Sociobiology. 2001;38:585–602. [Google Scholar]
- Anderson B. Adaptations to foliar absorption of faeces: a pathway in plant carnivory. Annals of Botany. 2005;95:757–761. doi: 10.1093/aob/mci082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzing DH. Foliar permeability and the absorption of minerals and organic nitrogen by certain tank Bromeliads. Botanical Gazette. 1970;131:23–31. [Google Scholar]
- Benzing DH, Ott DW, Friedman WE. Roots of Sobralia macrantha(Orchidaceae): structure and function of the velamen-exodermis complex. American Journal of Botany. 1982;69:608–614. [Google Scholar]
- Blatrix R, Bouamer S, Morand S, Selosse MA. Ant–plant mutualisms should be viewed as symbiotic communities. Plant Signaling and Behavior. 2009;4:554–556. doi: 10.4161/psb.4.6.8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croat TB. The flora of Barro Colorado Island. Palo Alto, CA: Stanford University Press; 1978. [Google Scholar]
- Di Giusto B, Anstett MC, Dounias E, McKey DB. Variation in the effectiveness of biotic defence: the case of an opportunistic ant–plant protection mutualism. Oecologia. 2001;129:367–375. doi: 10.1007/s004420100734. [DOI] [PubMed] [Google Scholar]
- Dressler RL. The orchids: natural history and classification. Cambridge, MA: Harvard University Press; 1981. [Google Scholar]
- Ellis AG, Midgley JJ. A new plant–animal mutualism involving a plant with sticky leaves and a resident hemipteran insect. Oecologia. 1996;106:478–481. doi: 10.1007/BF00329705. [DOI] [PubMed] [Google Scholar]
- Fiala B, Maschwitz U. Food bodies and their significance for obligate ant-associations in the tree genus Macaranga (Euphorbiaceae) Botanical Journal of the Linnean Society. 1992;110:61–75. [Google Scholar]
- Fischer RC, Richter A, Wanek W, Mayer V. Plants feed ants: food bodies of myrmecophytic Piper and their significance for the interaction with Pheidole bicornis ants. Oecologia. 2002;133:186–192. doi: 10.1007/s00442-002-1000-y. [DOI] [PubMed] [Google Scholar]
- Fischer RC, Wanek W, Richter A, Mayer V. Do ants feed plants? A N15 study of nitrogen fluxes from ants to plants in the mutualism of Pheidole and Piper. Journal of Ecology. 2003;91:126–134. [Google Scholar]
- Fisher BL. Facultative ant association benefits a Neotropical orchid. Journal of Tropical Ecology. 1992;91:126–134. [Google Scholar]
- Fisher BL, Zimmerman JK. Ant/orchid associations in the Barro Colorado national monument, Panama. Lindleyana. 1988;3:12–16. [Google Scholar]
- Fisher BL, Sternberg dSLL, Price D. Variation in the use of orchid extrafloral nectar by ants. Oecologia. 1990;83:263–266. doi: 10.1007/BF00317763. [DOI] [PubMed] [Google Scholar]
- Gay H. Animal-fed plants: an investigation into the uptake of ant-derived nutrients by the far-eastern epiphytic fern Lecanopteris Reinw. (Polypodiaceae) Biological Journal of the Linnean Society. 1993;50:221–233. [Google Scholar]
- Gaume L, McKey D, Terrin S. Ant–plant-homopteran mutualism: how the third partner affects the interaction between a plant-specialist ant and its myrmecophyte host. Proceedings of the Royal Society of London, B. 1998;265:569–575. [Google Scholar]
- Givnish TJ, Burkhardt EL, Happel RE, Weintraub JD. Carnivory in the bromeliad Brocchinia reducta, with a cost/benefit model for the general restriction of carnivorous plants to sunny, moist, nutrient-poor habitats. American Naturalist. 1984;124:479–497. [Google Scholar]
- Govaerts R, Pfahl J, Campacci MA, et al. World Checklist of Orchidaceae. The Board of Trustees of the Royal Botanic Gardens, Kew. 2010 Published online: http://www.kew.org/wcsp/ accessed 16 June 2010. [Google Scholar]
- Grime JP. Plant strategies, vegetative processes and ecosystem properties. Chichester, UK: Wiley-Liss; 2001. [Google Scholar]
- Heil M, McKey D. Protective ant–plant interactions as model systems in ecological and evolutionary research. Annual Review of Ecology Evolution and Systematics. 2003;34:425–453. [Google Scholar]
- Huxley CR. The ant plant Myrmecodia and Hydnophytum (Rubiaceae) and the relationship between their morphology, ant occupants, physiology and ecology. New Phytologist. 1978;80:231–268. [Google Scholar]
- Inselsbacher E, Cambui CA, Richter A, Stange CF, Mercier H, Wanek W. Microbial activities and foliar uptake of nitrogen in the epiphytic bromeliad Vriesea gigantea. New Phytologist. 2007;175:311–320. doi: 10.1111/j.1469-8137.2007.02098.x. [DOI] [PubMed] [Google Scholar]
- Janzen DH. Epiphytic myrmecophytes in Sarawak: mutualism through the feeding of plants by ants. Biotropica. 1974;6:237–259. [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London: Academic Press; 1989. [Google Scholar]
- Krol E, Plachno BJ, Adamec L, Stolarz M, Dziubinska H, Trebacz K. Quite a few reasons for calling carnivores ‘the most wonderful plants in the world. Annals of Botany109. 2012:47–67. doi: 10.1093/aob/mcr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Chapin FS III, Pons TL. Plant physiological ecology. New York, NY: Springer; 1998. [Google Scholar]
- Laube S, Zotz G. Which abiotic factors limit vegetative growth in a vascular epiphyte? Functional Ecology. 2003;17:598–604. [Google Scholar]
- Leigh EG, Jr., Rand AS, Windsor DM. The ecology of a tropical rainforest: seasonal rhythms and seasonal changes. Washington, DC: Smithsonian Institution Press; 1982. [Google Scholar]
- Leskovac V. Comprehensive enzyme kinetics. New York, NY: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- Markus M, Hess B, Ottaway JH, Cornish-Bowden A. The analysis of kinetic data in biochemistry: a critical evaluation of methods. FEBS Letters. 1976;63:225–230. doi: 10.1016/0014-5793(76)80100-7. [DOI] [PubMed] [Google Scholar]
- Oliveira PS, Rico-Gray V, Diaz-Castelazo C, Castillo-Guevara C. Interaction between ants, extrafloral nectaries and insect herbivores in neotropical coastal sand dunes: herbivore deterrence by visiting ants increases fruit set in Opuntia stricta (Cactaceae) Functional Ecology. 1999;13:623–631. [Google Scholar]
- Paracer S, Ahmadjian V. Symbiosis: an introduction to biological associations. 2nd edn. USA: Oxford University Press; 2000. [Google Scholar]
- Rico-Gray V. Tulane-University, New Orleans: USA; 1987. Schomburgkia tibicinis Batem. (Orchidaceae): effect of myrmecophyly on reproductive fitness. PhD Dissertation. [Google Scholar]
- Rico-Gray V, Barber JT, Thien LB, Ellgaard EG, Toney JJ. An unusual animal–plant interaction: feeding of Schomburgkia tibicinis (Orchidaceae) by ants. American Journal of Botany. 1989;76:603–608. [Google Scholar]
- Rico-Gray V, Oliveira PS. Chicago, IL: University of Chicago Press; 2007. The ecology and evolution of ant–plant interactions. [Google Scholar]
- Rico-Gray V, Thien LB. Effect of different ant species on reproductive fitness of Schomburgkia tibicinis (Orchidaceae) Oecologia. 1989;81:487–489. doi: 10.1007/BF00378956. [DOI] [PubMed] [Google Scholar]
- Rickson FR. Absorption of animal tissue breakdown products into a plant stem – the feeding of a plant by ants. American Journal of Botany. 1979;66:87–90. [Google Scholar]
- Sobrinho TG, Schoereder JH, Rodrigues LL, Collevatti RG. Ant visitation (Hymenoptera: Formicidae) to extrafloral nectaries increases seed set and seed viability in the tropical weed Triumfetta semitriloba. Sociobiology. 2002;39:353–368. [Google Scholar]
- Solano PJ, Dejean A. Ant-fed plants: comparison between three geophytic myrmecophytes. Botanical Journal of the Linnean Society. 2004;83:433–439. [Google Scholar]
- Stadler BS, Dixon AFG. Mutualism: ants and their insect partners. Cambridge: Cambridge University Press; 2008. [Google Scholar]
- Treseder KK, Davidson DW, Ehleringer JR. Absorption of ant-provided carbon dioxide and nitrogen by a tropical epiphyte. Nature. 1995;375:137–139. [Google Scholar]
- Tyler AC, McGlathery KJ, Macko SA. Uptake of urea and amino acids by the macroalgae Ulva lactuca (Chlorophyta) and Gracilaria vermiculophylla (Rhodophyta) Marine Ecology Progress Series. 2005;294:161–172. [Google Scholar]
- Von Wiren N, Gazzarini S, Gojon A, Frommer WB. The molecular physiology of ammonium uptake and retrieval. Current Opinions in Plant Biology. 2000;3:254–261. [PubMed] [Google Scholar]
- Wanek W, Pörtl K. Short time 15N uptake kinetics and nitrogen nutrition of bryophytes in a lowland rainforest, Costa Rica. Functional Plant Biology. 2008;35:51–62. doi: 10.1071/FP07191. [DOI] [PubMed] [Google Scholar]
- Windsor DM. Climate and moisture variability in a tropical forest: long term records from Barro Colorado Island, Panama. Washington, USA: Smithsonian Institution; 1990. [Google Scholar]
- Winkler U, Zotz G. Highly efficient uptake of phosphorus in epiphytic bromeliads. Annals of Botany. 2008;103:477–484. doi: 10.1093/aob/mcn231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanoviak SP, Berghof S, Linsenmair KE, Zotz G. Effects of an epiphytic orchid on arboreal ant community structure in Panama. Biotropica. 2011;43:731–737. [Google Scholar]
- Zotz G, Bermejo P, Dietz H. The epiphyte vegetation of Annona glabra on Barro Colorado Island, Panama. Journal of Biogeography. 1999;26:761–776. [Google Scholar]
- Zotz G, Hietz P. The physiological ecology of vascular epiphytes: current knowledge, open questions. Journal of Experimental Botany. 2001;52:2067–2078. doi: 10.1093/jexbot/52.364.2067. [DOI] [PubMed] [Google Scholar]
- Zotz G, Richter A. Changes in the carbohydrate and nutrient contents throughout a reproductive cycle indicate that phosphorus is a limiting nutrient in the epiphytic bromeliad, Werauhia sanguinolenta. Annals of Botany. 2006;97:745–754. doi: 10.1093/aob/mcl026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.